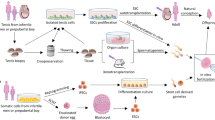
Abstract
Malfunction in spermatogenesis due to genetic diseases, trauma, congenital disorders or gonadotoxic treatments results in infertility in approximately 7% of males. The behavior of spermatogonial stem cells (SSCs) within three-dimensional, multifactorial, and dynamic microenvironment implicates a niche that serves as a repository for fertility, since can serve as a source of mature and functional male germ cells. Current protocols enable reprogramming of mature somatic cells into induced pluripotent stem cells (iPSCs) and their limited differentiation to SSCs within the range of 0–5%. However, the resulting human iPSC-derived haploid spermatogenic germ cell yield in terms of number and functionality is currently insufficient for transfer to infertility clinic as a therapeutic tool. In this article, we reviewed the evolution of experimental culture platforms and introduced a novel iPSCs-based approach for in vitro spermatogenesis based on a niche perspective bearing cellular, chemical, and physical factors that provide the complex arrangement of testicular seminiferous tubules embedded within a vascularized stroma. We believe that bioengineered organoids supported by smart bio-printed tubules and microfluidic organ-on-a-chip systems offer efficient, precise, personalized platforms for autologous pluripotent stem cell sources to undergo the spermatogenetic cycle, presenting a promising tool for infertile male patients with complete testicular aplasia.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide twelve to fifteen percent of couples are diagnosed with infertility, and approximately half of these cases are due to azoospermia in males. Azoospermia is characterized by the absence of sperm in the ejaculate and its incidence rate is 10–15% in infertile males [1]. Congenital anomalies and later acquisitions can lead to partial or permanent infertility in males. In case of low sperm or elongated/round spermatid production in testis, assisted reproductive techniques (ART), such as intracytoplasmic sperm injection (ICSI) and round spermatid injection (ROSI), offer a well-defined alternative to these patients. However, there is no effective treatment option for patients with germ cell aplasia or spermatogenic arrest in the earlier development than the round spermatid stage [2].
In limited preclinical studies, human induced pluripotent stem cells (hiPSCs) exhibited a significant treatment potential for male germ cell aplasia [3,4,5,6,7,8,9,10]. iPSCs reprogrammed from dermal fibroblasts [3, 11,12,13,14,15], cord blood, and keratinocytes [16] of healthy, non-obstructive azoospermic (NOA) men with an azoospermia factor 1 (AZF) deletion showed the capacity to differentiate into spermatogonial stem cells (SSCs), spermatogonial stem/progenitor cells, spermatocytes and spermatids in monolayer and air–liquid interface (ALI) culture setups. Those studies reported an efficiency of human SSCs (hSSCs) generation from iPSCs in culture systems with a wide variation ranging from 20 to 80%. The success rate of obtaining haploid germ cells is very limited, varying from 0 to 14%, and can therefore not be translated to ART applications.
These insufficient outputs underline the need for the development of 3D dynamic ex vivo setups that model the in vivo physical and chemical microenvironment of SSCs in testicular seminiferous tubules [17, 18]. The expected outcome is to enable progenitor germ cells to reach a number for spermatogenetic initiation sufficient for production of mature and functional sperm at satisfactory yields. Therefore, recent studies have tested in vitro spermatogenesis and testicular maturation in human samples using 2D and 3D culture systems, including monolayer, ALI, soft agar, testicular organoid, and microfluidic platforms (Table 1). The success of the present experimental designs mainly relies on niche-based concepts but faces long-term failure in the absence of a dynamic platform simulating the microvascular 3D physiology of the seminiferous tubules. Therefore, the development of a novel biomedical transdisciplinary approach that recapitulates the embryologic development of male germ stem/progenitor cells within the parenchymal and stromal testicular compartments, providing an effective cell-to-cell and cell-to-ECM crosstalk under appropriate physical conditions, is crucial for clinical translation. This article aims to provide a road map for researchers and health professionals working on male infertility to find a niche-based solution for the challenging progress of ex vivo spermatogenesis based on our group’s work history and other recent trials.
Method
A detailed literature review was carried out in PubMed using the keywords “in vitro spermatogenesis”, “testis organ culture”, “testis organoid”, “induced pluripotent stem cell/iPSC”, “Very small embryonic- like cells”, “male infertility”, and “germ cell aplasia”. The references section of this review includes the articles on in vitro human male germ cell differentiation published in English. The output of our group’s focused experience over ten years on stem cell niche, male infertility, and organ-on-a-chip models is cited and has been used to draw a guiding roadmap for future niche-based therapeutic concepts to male infertility research.
Male Factor Infertility and Germ Cell Aplasia
Infertility is the failure to achieve a successful pregnancy, as mentioned by the American Society for Reproductive Medicine (ASRM) in 2023 [19]. It is recommended that couples be followed up for infertility when pregnancy does not occur after 12 months of unprotected sexual intercourse if the female partner is under 35 years of age or six months of unprotected sexual intercourse if the female partner is 35 years of age or older. Infertility occurs in nearly 12–15% of couples and approximately 50% of these cases are due to male factors [20, 21]. Male factor infertility (MFI) is classified as congenital and acquired according to the point of origin in patients. Although MFI can be congenital with anomalies, such as bilateral absence of vas deferens, Sertoli Cell-Only (SCO) syndrome, cryptorchidism, Y chromosome microdeletion and Klinefelter syndrome, it can also be acquired at various stages of life due to environmental factors, such as testicular torsion, cancer treatment, trauma, multiple diseases or cytotoxic treatments [22, 23]. In case of bilateral absence or obstruction of the vas deferens, spermatogenesis continues normally, and functional sperms are produced in the testis; however, due to the lack of connection between the epididymis and urethra, the semen is devoid of sperm. As a routine practice of ART, sperm is collected from these patients by interventions, such as testicular sperm extraction (TESE) or micro-TESE followed by ICSI for pregnancy [24]. Similarly, cancer treatments, such as chemotherapy and radiotherapy, have gonadotoxic effects and can stop spermatogenesis in the testicle irreversibly. For male patients diagnosed with cancer in late adolescence or adulthood, mature sperm samples can be isolated before cytotoxic treatment within the scope of ART and pregnancy may be achieved through ICSI [25, 26]. There are several options in the clinic for treatment of infertility problems, where healthy spermatogenesis proceeds. However, these solutions are limited to patients having mature and functional sperm production.
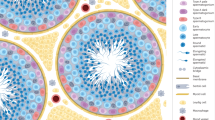
Congenital anomalies, such as SCO syndrome, cryptorchidism, Klinefelter syndrome or Y chromosome-microdeletion [27] and acquired causes, such as testicular trauma, childhood cancer treatment, testis torsion, autoimmune response and viral infections [28] cause arrests in the early stages of spermatogenesis before spermatid or complete germ cell loss in testes (Fig. 1).
The patients face permanent infertility since no improved treatment procedure can provide pregnancy. Since the cellular and physical integrity of the germ cell niche is impaired in male patients with germ cell aplasia, regardless of etiological factors, they cannot conceive a biological child. Because the spermatogenic cycle cannot be initiated, these males are not able to produce mature sperm or even late-stage spermatids. Recently, in vitro spermatogenesis is targeted with niche simulation using germ stem cell, organ, organoid or bioprinting-based culture platforms for patients with a damaged/impaired testicular milieu.
The Spermatogonial Stem Cell Niche for in vitro Spermatogenesis
The spermatogonial stem cell lineage localizes and acts within a unique microenvironment, resembling that of somatic stem cells [29, 30]. The testicular microenvironment of the SSCs comprises several accessory cellular and extracellular matrix elements which interact within a physical and chemical milieu. The overall zone that encourages the self-renewal of male germ stem cells to preserve spermatogenic cell pool at adequate size, is referred to as “niche”. Since spermatogonial stem cells constitute only 22% of the niche in humans [31] and 0.01- 0.02% in mice [32], the accessory elements comprise the major fraction. The spermatogonial stem cell niche (Fig. 1. a) is placed at the seminiferous tubules lined by a germinal epithelium that forms the parenchyma and takes a part of the stroma between the tubules in the testes [33]. The spermatogenic stem cells remain undifferentiated due to lack of androgenic endocrine induction but maintain the germ cell pool within the human testicular cords until puberty. The somatic Sertoli cells (SCs) assist germ cell renewal and provide the blood-testis barrier during childhood.
By the onset of the puberty, spermatogenic cell series that start from the basement membrane (BM) present a stratified epithelial-like tissue when they undergo differentiation with the support of somatic SCs. The interstitial or stromal testicular compartment comprises Leydig cells (LCs), peritubular myoid cells (PMCs), macrophages and blood vessels. Sertoli cells are highly polarized and cytoplasmic projections of SCs laterally extend to the lumen of seminiferous tubules while their basal region is located on the BM [33, 34]. The somatic cells support self-renewal and survival of SSCs in many ways. Sertoli cells form a blood-testis barrier and produce glial cell line-derived neurotrophic factor (GDNF) and fibroblast growth factor 2 (FGF2) to provide structural and homeostatic support to the SSC pool. Leydig cells are androgenic cells after puberty. They are activated by luteinizing hormone (LH) and secrete testosterone, which is responsible for stimulation of spermatogenic lineage cells to the terminal stage. Both LCs and PMCs produce colony-stimulating factor 1 (CSF1) to conserve SSC self-renewal [29, 34, 35]. Experimental ex vivo testicular niche setups require the presence of both parenchymal and stromal compartments that physiologically act in harmony with neural, paracrine, endocrine, and vascular functions to obtain fertile spermatozoa.
Spermatogenesis comprises consecutively spermatogonial, spermatocyte and spermatid phases (Fig. 2). One cycle of human spermatogenesis lasts about 64 days [36] and starts with the spermatogonial phase due to SSC mitosis into two daughter cells [37]. One of the daughter cells continues mitosis to preserve self-renewal of SSCs and the other one begins differentiation [37]. The new cycle of spermatogenesis can start when the previous cycle is completed [36]. The daughter cells of the SSCs are classified into three types based on their nuclear histochemical staining intensity as type Adark, type Apale and type B spermatogonia. Type Adark spermatogonia are responsible for the SSC pool and undergo mitotic division. Type Apale spermatogonia differentiate to form type B spermatogonia [38,39,40]. Type Adark and B spermatogonia are connected to each other with cytoplasmic bridges and these connections continue until the spermatid phase. In the spermatocyte phase firstly, type B spermatogonia mitotically divide to generate primary spermatocytes with a normal chromosome number. Whereas spermatogenesis occurs in the basal region, the remainder occurs in the lumen of the seminiferous tubules. Then, primary spermatocytes (2n) undergo their first meiotic division in order to obtain haploid DNA (n) while forming secondary spermatocytes (Fig. 2). After the second meiotic division, haploid spermatids are produced [38, 41]. Finally, spermatids undergo morphological remodeling to form mature sperms in the spermatid phase (Fig. 2). In this phase, spermatids undergo Golgi, cap, acrosome, and maturation phases, after which spermatogenesis is completed. The mature sperms are released from the basal region of SCs to seminiferous tubules with the help of PMCs [38].
In the last decade, the presence of very small embryonic-like cells (VSELs) has been reported in both the pre- and post-pubertal human testicular microenvironment [42,43,44]. The spherical shaped VSELs are overlooked in many studies due to their very small-size (range of 2- 6 µm diameter) [45]. However several groups define the human VSELs located along the basal lamina as similar to the SSCs, but more primitive due to the presence of the pluripotency markers, such as OCT4, NANOG, SOX2, LIN-/CD45-/CD133 + , SSEA4 + , SSEA1 + , and a high nucleocytoplasmic ratio at the ultrastructural level [44, 46]. Human testicular primordial germ cell like VSELs are physiologically quiescent, however they can rapidly divide and give rise to SSCs when exposed to stress-inducing conditions. This may present a potential tool for the SSC enrichment tools [45, 47]. Ex vivo spermatogenesis frameworks require the completion of the whole orchestrated process of germ stem cell differentiation and the generation of a sufficient number of functional sperms in order to achieve efficient fertility incomes in the clinic.
The Evolution of Testicular Culture Platforms
To create new treatment options for congenital and acquired male infertility, there is a need for systems that model the 3D hSSC niche with active neural inputs, paracrine/endocrine secretome and a continuous vascular flow of the testis. When spermatogenesis cannot start during puberty because of damaged SSCs and other cellular and extracellular niche components due to chemotherapy and/or radiotherapy for prepubertal cancers, clinically permanent infertility may be observed in half of these patients. In countries with fertility preservation programs, testicular biopsies are usually taken from patients before gonadotoxic therapy, depending on their parents' approval. The aim is to protect the existing SSCs via cryopreservation. However, in a small population of patients with a damaged testicular microenvironment due to cancer therapy, fertility cannot be achieved from the cryopreserved SSCs [25, 26, 48]. Therefore, in vitro expansion and differentiation of the isolated SSC pool is crucial for restoration of fertility. However, because the self-renewal and spermatogenic differentiation process is highly niche dependent for SSCs, this may hinder the success of ex vivo spermatogenesis.
Monolayer stem cell cultures in culture plates coated with Matrigel/Fibronectin or a feeder layer (i.e. mouse embryonic fibroblasts) have been shown to provide short-term maintenance for rodent [49] and human [50] SPC lineages. A β-estradiol-supported monolayer germ stem cell-Sertoli cell co-culture system increased hSSC number by physical contact and intercellular crosstalk with SCs and extrinsic Leydig cell-based chemical factor supplementation, when compared to monoculture of SSCs until day 7 [49]. Addition of epididymal white adipose tissue (EWAT)-derived leptin showed limited performance by increasing colony formation and real-time proliferation rate when used in to expand newborn mouse SPCs that are cultured on mouse embryonic fibroblasts until day 7 [51]. The co-culture of allogeneic adult EWAT with newborn mouse prepubertal testicular strips on an ALI system provided a longer survival and enrichment of SPCs until day 14. EWAT secretome also supported the differentiation of SSCs up till the round spermatid stage in a diffusion permeable static organ culture setup. The addition of allogeneic mouse bone marrow mesenchymal stem cells (BMSCs) to the ALI co-culture system enhanced the success of IVS in terms of SSC proliferation and SCP3 and acrosin-positive haploid germ cell formation, which increased up to 28 days. Considering the ALI platform’s ability to simulate SSC niche, allogeneic BMSCs having a similar embryonic origin are able to replace and support SCs that are essential for the engraftment and differentiation of spermatogenic cells. Thus, they preserve the integrity of tight junctions and exert a paracrine support by GDNF, FGF, stem cell factor (SCF), TPO and vascular endothelial growth factor (VEGF) [52]. Human fetal gonad strips obtained from 12 to 19-week-old abortus material were maintained in this ALI platform for 50 days and allowed the differentiation of haploid cells, but at a limited efficiency, ranging between 0.07 to 9.83%. When ROSI was performed with these haploid cells, resulting embryos were shown to develop until the blastocyst stage [53]. Air–liquid interphase cultures provide an inadequate yield of haploid germ cells or complete absence of haploid germ cell production after 5 to 139 days of culture of human testicular biopsies from prepubertal cancer patients [54,55,56]. When GPR125 positive SSCs and SCs isolated from testicular biopsies from 80 post-pubertal obstructive azoospermia patients with normal sperm production were embedded into Matrigel and cultured via the ALI platform for 20 days, the system yielded haploid cells with 0.8 to 17.9% efficiency [57]. Spermatogonial stem cells obtained from testicular biopsies taken from 14 post-pubertal (21- 41 years old) male patients with non‐obstructive azoospermia (NOA) were cultured in agarose for two weeks in the 3D soft agar culture system. 3D soft agar conditions increased hSSCs two times and haploid germ cells increased 2–3 times compared to 2D monolayer culture [58], clearly demonstrating a need for novel organ culture designs.
In the last decade, organoid, bioprinter-based seminiferous tubule and testicular cell aggregate culture systems have replaced monolayers for simulation of the healthy spermatogenic microenvironment outside the body and to obtain mature and functional sperm from these patients (Table 1). Organoids can be generated through culture of pluripotent or primary niche cells. The aggregation of those cells resulting in adhesion, self-organization and differentiation into 3D cell masses might represent the corresponding organ’s functional morphology [59]. A 5-day culture of organoids obtained from prepubertal pig, macaque, and mouse testicular samples under static conditions resulted in adhesive connections of SCs and PMCs but was unable to sustain the germ cell pool [60] (Table 1). Post-pubertal healthy donor testis organoids yielded hSSCs with 52% efficiency after 23 days of culture, while the yield of haploid cells was limited to 0.2% [61]. Bioprinting is the fabrication of complex biological constructs regarding cellular/extracellular content and tissue topography, including tube-shaped architecture of seminiferous tubules [62]. The cell suspension of SSC/Sertoli/Leydig/endothelial cells obtained from a testicular biopsy of an adult SCO patient with partial germ cell aplasia was seeded into a 3D bio-printed seminiferous tubule or aggregated as an organoid, followed by culture for 12 days. The study reported a fourfold increase in the number of ID4 positive hSSCs, a twofold increase in SCP3 positive spermatocytes and a decrease in the number of PRM2 positive spermatids in the bio-printed seminiferous tubule compared to the organoid [63]. Taken together, 3D smart bioengineered materials present promising tools, when combined with amassed cell-sourced organoids mimicking embryologic nest topography.
In conclusion, ex vivo culture conditions have evolved from basic monolayer SSC cultures containing only the stem cells and an artificial chemical microenvironment generated by supplementation, devoting of physical contact, and crosstalk with neighbor cells within the SSC niche to static organ culture systems [51]. These systems comprise ALI and hanging drop setups and the bioengineering product-based scaffolds. Briefly ALI permits a homogenous diffusion through a biphasic compartment, while the hanging drop system avoids the damage by immersion of the organ strip in growth factor containing media [10, 41, 64]. The bioengineering products such as natural and synthetic (alginate) polymers that make 3D immersion setups replace the ECM of the stromal compartment of the niche model and mimic the testicular microenvironment ex vivo. These static setups caring seminiferous tubule strips are highly similar to the SSC niche in terms of collecting spermatogenic cells, accessory cells (Sertoli cell, Leydig cell, PMC, etc.), secretome based on physical contact and intercellular crosstalk (chemical factors). However, they lack several crucial physical elements such as vascular flow, shear stress, O2 gradient, and equal distribution of nutrients to growing seminiferous tubules. These shortcomings necessitated the development of real organoids and organ-on-a-chip systems that involve differentiation of pluri/multipotent stem cells within a dynamic 3D milieu. Our group recently designed and generated a factitious microfluidic compartment mimicking not only cellular and chemical factors, but also the physical elements in the SSC niche with a pumpless flow in microchannels, polydimethylsiloxane (PDMS)-controlled O2 gradient and diffusion-based nutrient distribution to the seminiferous tubules through an array of rectangular micropillars and bilateral medium perfusion around an organ chamber [17, 65]. In the microfluidic device designed with a holistic niche concept and supported by allogeneic BMSCs secretome, the duration of the culture period was increased to 42 days, and thus the artificial niche environment could be maintained throughout a complete cycle of mouse spermatogenesis, which lasts for 34 days under in vivo conditions. The actual success rate achieved from current culture systems may encourage infertility professionals to treat patients who already have a healthy SSC niche and/or healthy SSC population, but patients with complete germ cell aplasia still require a novel germ cell source. For this reason, a few groups, including ours, are currently conducting studies on the differentiation of human ESCs and iPSCs from autologous sources into SSC and haploid germ cells.
Pluripotent Stem Cell-Based Culture Technology as an Opportunity to Restore Germ Cell Aplasia
Currently, methods for in vitro generation of male germ cells have generally been based on pluripotent stem cells (PSCs), due to their plasticity (Fig. 3). Both ESCs and iPSCs are classified as pluripotent stem cells. ESCs are collected from the inner cell mass of the blastocyst stage and have the full potential to differentiate into cells from ectoderm, mesoderm and endodermal germ layers [74]. However, due to ethical and allogenic restrictions their use is limited, even though their differentiation potential makes ESCs a good candidate for in vitro male germ cell differentiation [75,76,77]. iPSCs are reprogrammed from somatic cells and gain a pluripotent profile, similar to ESCs [78]. There are two ways to conduct this process: chemically induced supplements or vectors, which can either be non-integrating or integrating. The efficiency of integrating viral vectors is higher when compared to induction via chemicals and the use of non-integrating vectors. However, viral genomic integration into the host cell genome may cause unpredictable and permanent mutagenesis [79]. Since VSELs are reported to be adult testicular primitive primordial germ cells or putative PSCs [42, 45, 47, 80] they may therefore be worth further assessment, as an alternative source for the infertility studies [47]. In this context, recently non-integrating vectors presenting higher safety profiles have come forward in iPSC manufacturing technology and could be used in autogenic or allogeneic stem cell-based therapies, including male fertility approaches [81, 82].
The schematic illustration of the current in vitro male germ cell differentiation protocols from iPSCs. hiPSCs: Human induced pluripotent stem cells, hESCs: Human embryonic stem cells, hPGCs: Human primordial germ cells, hSSCs: Human spermatogonial stem cells, hSPCs: Human spermatogonial progenitor cells, VPA: Valproic acid, BSA: Bovine serum albumin, GDNF: Glial cell line-derived neurotrophic factor, FGF: Fibroblast growth factor, RA: Retinoic acid, LIF: Leukemia inhibitory factor, FRSK: Forskolin, FBS: Fetal bovine serum, BMP: Bone morphogenetic protein, SCF: Stem cell factor, EGF: Epidermal growth factor, AA: Activin A, ITS: Insulin-Transferrin-Selenium, FSH: Follicle stimulating hormone
Both human ESCs (hESCs) and hiPSCs have been differentiated to male germ cells by various in vitro methods, but with a very low yield (Fig. 3, Table 2) [3, 5, 11,12,13, 15, 83]. Human iPSCs are induced with secretomes of Sertoli cells (bFGF, GDNF), EWAT (palmitic acid, palmitoleic acid, linoleic acid, valproic acid), putrescine, insulin, transferrin, sodium selenite, and vitamin C to differentiate into SSCs [3, 11, 84, 85]. Human iPSCs have been stimulated with GDNF, retinoic acid (RA), bone morphogenetic protein (BMP) 4, BMP8b, leukemia inhibitory factor (LIF), forskolin (FRSK) and R115866 to differentiate to haploid germ cells [5, 12]. In a recent study, commercial hESC lines and NOA-patient primary dermal fibroblast-derived iPSCs are evaluated for their differentiation capacity into the SSCs using valproic acid (VPA) and vitamin C. Human PSCs are induced by knockout serum replacement (KSR), GlutaMAX™, Insulin-Transferrin-Selenium (ITS), lipid mix, GDNF, bFGF, VPA and vitamin C for 12 days. Application of VPA and vitamin C together increases PLZF + , GPR125 + , GFRa1 + , ID4 + SSCs and SYCP3 + , Acrosin + , TNP1 + haploid germ cells [11]. These studies suggest iPSCs may be a promising source of SSC and haploid germ cells, however, the haploid germ cell yield from iPSCs is currently limited to a range of 0–5% in monolayer, static culture systems.
Taken together, monolayer culture has been widely used in studies targeting in vitro spermatogenesis from iPSCs, but only two studies used cell aggregates and EB-based 3D platforms [83, 84]. We foresee that the experience we have gained from our niche concept-based work history will serve as a crucial milestone to increase the yield of SSCs and haploid germ cells from iPSCs.
Conclusion
Repeated clinical failure has directed the current strategy to ensure functional testicular maturation, SSC proliferation and spermatogenesis by in vitro culture of prepubertal testicular samples containing hSSCs and immature Sertoli, Leydig cells and myoid cells. From another perspective, spermatogenesis might be arrested before germ cells become haploid and gain fertilization potential, due to impairment of niche components and blood supply disruption such as in cases of testicular torsion, trauma, varicocele and infection in adulthood [22]. The overall preclinical experience reveals the capacity of 3D culture platforms to support in vitro spermatogenesis from 5 to 139 days. The 3D organ, organoid, and bio-printed testicular static cultures give limited yields and differentiation capacities with insufficient numbers of post-meiotic germ cells. The evolution from monolayer to 3D in vitro spermatogenesis platforms improved the yields to some extent, especially when microfluidic flow enforced allogeneic BMSC secretome was used within a 3D dynamic tubular topography.
However, generation of a continuous supply with a high yield of fertile spermatozoon requires reprogramming of pluripotent stem cells. Recently, pluripotent stem cell-based culture technology has supported the possibilities to restore somatic and also germ cell pools. Although autologous iPSCs may present a promising tool for SSCs this method currently only gives a very limited yield of haploid germ cell production in the range of 0–5%, underlining that the present culture systems are insufficient for clinical translation of treatment for germ cell aplasia. We propose that stem cell-assisted microfluidic-based platforms could be used to overcome this boundary in the near future, provided that smart bioengineering meets the micro-physiological needs. Besides functionality, the genetic stability of ex vivo differentiated haploid cells and the embryos needs to be validated before transferring these systems to the clinic. Our evolutionary niche-based road map could guide future translation of stem cell therapeutics into the male infertility clinic and may be used as a precise personalized tool by exploring the possibilities of 3D organ-on-a-chip platforms.
Data Availability
Not applicable.
Abbreviations
- AA:
-
Activin A
- ALI:
-
Air-Liquid Interface
- ART:
-
Assisted Reproductive Techniques
- ASRM:
-
American Society for Reproductive Medicine
- BM:
-
Basal Membrane
- BMP:
-
Bone Morphogenetic Protein
- CSF1:
-
Colony-Stimulating Factor 1
- EGF:
-
Epidermal Growth Factor
- ESCs:
-
Embryonic Stem Cells
- FBS:
-
Fetal Bovine Serum
- FGF:
-
Fibroblast Growth Factor
- FRSK:
-
Forskolin
- FSH:
-
Follicle Stimulating Hormone
- GDNF:
-
Glial Cell Line-Derived Neurotrophic Factor
- ICSI:
-
Intracytoplasmic Sperm Injection
- iPSCs:
-
Induced Pluripotent Stem Cells
- ITS:
-
Insulin-Transferrin-Selenium
- KSR:
-
Knockout Serum Replacement
- LCs:
-
Leydig Cells
- LIF:
-
Leukemia Inhibitory Factor
- MFI:
-
Male Factor Infertility
- NOA:
-
Non-Obstructive Azoospermia
- PGCs:
-
Primordial Germ Cells
- PMCs:
-
Peritubular Myoid Cells
- PSCs:
-
Pluripotent Stem Cells
- RA:
-
Retinoic Acid
- ROSI:
-
Round Spermatid Injection
- SCs:
-
Sertoli Cells
- SCF:
-
Stem Cell Factor
- SCO:
-
Sertoli Cell-Only
- SPCs:
-
Spermatogonial Progenitor Cells
- SSCs:
-
Spermatogonial Stem Cells
- TESE:
-
Testicular Sperm Extraction
- VPA:
-
Valproic Acid
- VSELs:
-
Very Small Embryonic- Like Cells
References
Omolaoye, T. S., Hachim, M. Y., & du Plessis, S. S. (2022). Using publicly available transcriptomic data to identify mechanistic and diagnostic biomarkers in azoospermia and overall male infertility. Scientific Reports, 12(1), 2584. https://doi.org/10.1038/s41598-022-06476-1
Leslie, S. W., Mejias, S. G., & Ramphul, K. (2023). Sertoli cell–only syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
Easley, C. A., IV., Phillips, B. T., McGuire, M. M., Barringer, J. M., Valli, H., Hermann, B. P., Simerly, C. R., Rajkovic, A., Miki, T., & Orwig, K. E. (2012). Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Reports, 2(3), 440–446.
Ishikura, Y., Yabuta, Y., Ohta, H., Hayashi, K., Nakamura, T., Okamoto, I., Yamamoto, T., Kurimoto, K., Shirane, K., Sasaki, H., & Saitou, M. (2016). In vitro derivation and propagation of spermatogonial stem cell activity from mouse pluripotent stem cells. Cell Reports, 17(10), 2789–2804. https://doi.org/10.1016/j.celrep.2016.11.026
Eguizabal, C., Montserrat, N., Vassena, R., Barragan, M., Garreta, E., Garcia-Quevedo, L., Vidal, F., Giorgetti, A., Veiga, A., & Izpisua Belmonte, J. C. (2011). Complete meiosis from human induced pluripotent stem cells. Stem Cells, 29(8), 1186–1195. https://doi.org/10.1002/stem.672
Nolte, J., Michelmann, H. W., Wolf, M., Wulf, G., Nayernia, K., Meinhardt, A., Zechner, U., & Engel, W. (2010). PSCDGs of mouse multipotent adult germline stem cells can enter and progress through meiosis to form haploid male germ cells in vitro. Differentiation, 80(4–5), 184–194.
Yang, S., Bo, J., Hu, H., Guo, X., Tian, R., Sun, C., Zhu, Y., Li, P., Liu, P., Zou, S., Huang, Y., & Li, Z. (2012). Derivation of male germ cells from induced pluripotent stem cells in vitro and in reconstituted seminiferous tubules. Cell Proliferation, 45(2), 91–100. https://doi.org/10.1111/j.1365-2184.2012.00811.x
Zhu, Y., Hu, H.-L., Li, P., Yang, S., Zhang, W., Ding, H., Tian, R.-H., Ning, Y., Zhang, L.-L., & Guo, X.-Z. (2012). Generation of male germ cells from induced pluripotent stem cells (iPS cells): An in vitro and in vivo study. Asian journal of andrology, 14(4), 574.
Wang, H., Xiang, J., Zhang, W., Li, J., Wei, Q., Zhong, L., Ouyang, H., & Han, J. (2016). Induction of germ cell-like cells from porcine induced pluripotent stem cells. Scientific reports, 6, 27256. https://doi.org/10.1038/srep27256
Adriansyah, R. F., Margiana, R., Supardi, S., & Narulita, P. (2023). Current progress in stem cell therapy for male infertility. Stem Cell Reviews and Reports, 19(7), 2073–2093. https://doi.org/10.1007/s12015-023-10577-3
Wang, X., Qu, M., Li, Z., Long, Y., Hong, K., & Li, H. (2021). Valproic acid promotes the in vitro differentiation of human pluripotent stem cells into spermatogonial stem cell-like cells. Stem Cell Research & Therapy, 12(1), 553. https://doi.org/10.1186/s13287-021-02621-1
Zhao, Y., Ye, S., Liang, D., Wang, P., Fu, J., Ma, Q., Kong, R., Shi, L., Gong, X., & Chen, W. (2018). In vitro modeling of human germ cell development using pluripotent stem cells. Stem cell reports, 10(2), 509–523.
Panula, S., Medrano, J. V., Kee, K., Bergström, R., Nguyen, H. N., Byers, B., Wilson, K. D., Wu, J. C., Simon, C., Hovatta, O., & Reijo Pera, R. A. (2011). Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Human Molecular Genetics, 20(4), 752–762. https://doi.org/10.1093/hmg/ddq520
Ramathal, C., Durruthy-Durruthy, J., Sukhwani, M., Arakaki Joy, E., Turek Paul, J., Orwig Kyle, E., & Reijo Pera Renee, A. (2014). Fate of iPSCs derived from azoospermic and fertile men following xenotransplantation to murine seminiferous tubules. Cell Reports, 7(4), 1284–1297. https://doi.org/10.1016/j.celrep.2014.03.067
Durruthy Durruthy, J., Ramathal, C., Sukhwani, M., Fang, F., Cui, J., Orwig, K. E., & Reijo Pera, R. A. (2014). Fate of induced pluripotent stem cells following transplantation to murine seminiferous tubules. Human Molecular Genetics, 23(12), 3071–3084. https://doi.org/10.1093/hmg/ddu012
Eguizabal, C., Montserrat, N., Veiga, A., & Belmonte, J. C. I. (2013). Dedifferentiation, transdifferentiation, and reprogramming: future directions in regenerative medicine. Seminars in Reproductive Medicine, 31, 082–094. Thieme Medical Publishers.
Önen, S., Atik, A. C., Gizer, M., Köse, S., Yaman, Ö., Külah, H., & Korkusuz, P. (2023). A pumpless monolayer microfluidic device based on mesenchymal stem cell-conditioned medium promotes neonatal mouse in vitro spermatogenesis. Stem Cell Research & Therapy, 14(1), 127. https://doi.org/10.1186/s13287-023-03356-x
Önen, S., Köse, S., Yersal, N., & Korkusuz, P. (2022). Mesenchymal stem cells promote spermatogonial stem/progenitor cell pool and spermatogenesis in neonatal mice in vitro. Scientific reports, 12(1), 11494. https://doi.org/10.1038/s41598-022-15358-5
Gracia, C., Anderson, J., Amato, P., Flyckt, R., Hansen, K., Hill, M., Jindal, S., Kalra, S., Jain, T., Pier, B., Thomas, M., Robins, J., Shannon, C. N., Steiner, A., Tanrikut, C., & Yauger, B. (2023). Definition of infertility: A committee opinion. Fertility and Sterility, 120(6), 1170–1170.
Key Statistics for Childhood Cancers. (2022) https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Accessed 04.08.2022
Schlegel, P. N., Sigman, M., Collura, B., De Jonge, C. J., Eisenberg, M. L., Lamb, D. J., Mulhall, J. P., Niederberger, C., Sandlow, J. I., Sokol, R. Z., Spandorfer, S. D., Tanrikut, C., Treadwell, J. R., Oristaglio, J. T., & Zini, A. (2021). Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline Part I. Journal of Urology, 205(1), 36–43. https://doi.org/10.1097/ju.0000000000001521
Dimitriadis, F., Adonakis, G., Kaponis, A., Mamoulakis, C., Takenaka, A., Sofikitis, N. (2017). Pre-testicular, testicular, and post-testicular causes of male infertility. In M. Simoni, I. Huhtaniemi (Eds.), Endocrinology of the Testis and Male Reproduction. Endocrinology. (pp. 1–47). Springer, Cham. https://doi.org/10.1007/978-3-319-29456-8_33-1
Cao, J., Zhao, X., Qin, Z., Lv, S., Du, L., Liu, Z., Fan, L., & Bo, H. (2024). Single cell map of human azoospermia testis caused by cyclophosphamide chemotherapy. Scientific Data, 11(1), 163. https://doi.org/10.1038/s41597-024-02938-5
Lin, C. H., & Huang, T. Y. (2020). Congenital bilateral absence of the vas deferens (CBAVD) with bilaterally present seminal vesicles. Urol Case Rep, 31, 101131. https://doi.org/10.1016/j.eucr.2020.101131
ASRM. (2019). Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertility and Sterility, 112(6), 1022–1033. https://doi.org/10.1016/j.fertnstert.2019.09.013
ASRM. (2018). Fertility preservation and reproduction in patients facing gonadotoxic therapies: an ethics committee opinion. Fertility and Sterility, 110(3), 380–386. https://doi.org/10.1016/j.fertnstert.2018.05.034
Wyrwoll, M. J., van der Heijden, G. W., Krausz, C., Aston, K. I., Kliesch, S., McLachlan, R., Ramos, L., Conrad, D. F., O’Bryan, M. K., Veltman, J. A., & Tüttelmann, F. (2023). Improved phenotypic classification of male infertility to promote discovery of genetic causes. Nature Reviews Urology. https://doi.org/10.1038/s41585-023-00816-0
Sharma, A., Minhas, S., Dhillo, W. S., & Jayasena, C. N. (2021). Male infertility due to testicular disorders. Journal of Clinical Endocrinology and Metabolism, 106(2), e442–e459. https://doi.org/10.1210/clinem/dgaa781
Köse, S., Yersal, N., Önen, S., Korkusuz, P. (2018). Comparison of hematopoietic and spermatogonial stem cell niches from the regenerative medicine aspect. Adv Exp Med Biol, 1107, 15–40. https://doi.org/10.1007/5584_2018_217
Köse, S., Aerts-Kaya, F., Köprü, Ç. Z., Nemutlu, E., Kuşkonmaz, B., Karaosmanoğlu, B., Taşkıran, E. Z., Altun, B., Uçkan Çetinkaya, D., & Korkusuz, P. (2018). Human bone marrow mesenchymal stem cells secrete endocannabinoids that stimulate in vitro hematopoietic stem cell migration effectively comparable to beta-adrenergic stimulation. Experimental Hematology, 57, 30-41.e31. https://doi.org/10.1016/j.exphem.2017.09.009
Bashiri, Z., Gholipourmalekabadi, M., Khadivi, F., Salem, M., Afzali, A., Cham, T.-C., & Koruji, M. (2023). In vitro spermatogenesis in artificial testis: current knowledge and clinical implications for male infertility. Cell and Tissue Research, 394, 1–29.
Ibtisham, F., & Honaramooz, A. (2020). Spermatogonial stem cells for ın vitro spermatogenesis and ın vivo restoration of fertility. Cells, 9(3), 745. https://doi.org/10.3390/cells9030745
Salem, M., Khadivi, F., Javanbakht, P., Mojaverrostami, S., Abbasi, M., Feizollahi, N., Abbasi, Y., Heidarian, E., & Rezaei Yazdi, F. (2023). Advances of three-dimensional (3D) culture systems for in vitro spermatogenesis. Stem Cell Research & Therapy, 14(1), 262.
Oliver, E., & Stukenborg, J. B. (2020). Rebuilding the human testis in vitro. Andrology, 8(4), 825–834.
Liu, Z.-j, Liu, Y.-h, Huang, S.-y, & Zang, Z.-J. (2021). Insights into the regulation on proliferation and differentiation of stem leydig cells. Stem Cell Reviews and Reports, 17(5), 1521–1533. https://doi.org/10.1007/s12015-021-10133-x
Eisenberg, M. L., Esteves, S. C., Lamb, D. J., Hotaling, J. M., Giwercman, A., Hwang, K., & Cheng, Y. S. (2023). Male infertility. Nature Reviews Disease Primers, 9(1), 49. https://doi.org/10.1038/s41572-023-00459-w
Witherspoon, L., & Flannigan, R. (2022). It puts the T’s in fertility: Testosterone and spermatogenesis. International Journal of Impotence Research, 34(7), 669–672. https://doi.org/10.1038/s41443-022-00531-1
Pawlina, W., & Ross, M. H. (2018). Histology: A text and atlas: With correlated cell and molecular biology. Lippincott Williams & Wilkins.
Cannarella, R., Condorelli, R. A., Mongioì, L. M., La Vignera, S., & Calogero, A. E. (2020). Molecular biology of spermatogenesis: Novel targets of apparently idiopathic male infertility. International Journal of Molecular Sciences, 21(5), 1728.
Zhou, S., Feng, S., Qin, W., Wang, X., Tang, Y., & Yuan, S. (2021). Epigenetic regulation of spermatogonial stem cell homeostasis: from DNA methylation to histone modification. Stem Cell Reviews and Reports, 17(2), 562–580. https://doi.org/10.1007/s12015-020-10044-3
Robinson, M., Sparanese, S., Witherspoon, L., & Flannigan, R. (2023). Human in vitro spermatogenesis as a regenerative therapy — where do we stand? Nature Reviews Urology. https://doi.org/10.1038/s41585-023-00723-4
Bhartiya, D., Anand, S., Patel, H., & Parte, S. (2017). Making gametes from alternate sources of stem cells: Past, present and future. Reproductive Biology and Endocrinology, 15, 1–14.
Bhartiya, D., Mohammad, S. A., Singh, P., Sharma, D., & Kaushik, A. (2022). GFP tagged VSELs help delineate novel stem cells biology in multiple adult tissues. Stem Cell Reviews and Reports, 18(5), 1603–1613.
Bhartiya, D., Raouf, S., Pansare, K., Tripathi, A., Tripathi, A. (2024). Initiation of cancer: The journey from mutations in somatic cells to epigenetic changes in tissue-resident VSELs. Stem Cell Rev Rep. https://doi.org/10.1007/s12015-024-10694-7
Bhartiya, D., Singh, P., Sharma, D., & Kaushik, A. (2022). Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues. Stem Cell Reviews and Reports, 18(5), 1718–1727.
Bhartiya, D., Anand, S., & Kaushik, A. (2019). Pluripotent very small embryonic-like stem cells co-exist along with spermatogonial stem cells in adult mammalian testis. Human Reproduction Update, 26(1), 137–138. https://doi.org/10.1093/humupd/dmz030
Bhartiya, D., Shaikh, A., Anand, S., Patel, H., Kapoor, S., Sriraman, K., Parte, S., & Unni, S. (2017). Endogenous, very small embryonic-like stem cells: Critical review, therapeutic potential and a look ahead. Human Reproduction Update, 23(1), 41–76.
Siegel, R. L., Miller, K. D., & Fuchs, H. E. (2022). Jemal A (2022) Cancer statistics. CA: A Cancer Journal for Clinicians, 72(1), 7–33. https://doi.org/10.3322/caac.21708
Tao, K., Sun, Y., Chao, Y., Xing, L., Leng, L., Zhou, D., Zhu, W., & Fan, L. (2021). β-estradiol promotes the growth of primary human fetal spermatogonial stem cells via the induction of stem cell factor in Sertoli cells. Journal of Assisted Reproduction and Genetics, 38(9), 2481–2490. https://doi.org/10.1007/s10815-021-02240-y
Medrano, J. V., Rombaut, C., Simon, C., Pellicer, A., & Goossens, E. (2016). Human spermatogonial stem cells display limited proliferation in vitro under mouse spermatogonial stem cell culture conditions. Fertility and Sterility, 106(6), 1539-1549.e1538. https://doi.org/10.1016/j.fertnstert.2016.07.1065
Yersal, N., Köse, S., Horzum, U., Özkavukcu, S., Orwig, K. E., & Korkusuz, P. (2020). Leptin promotes proliferation of neonatal mouse stem/progenitor spermatogonia. Journal of Assisted Reproduction and Genetics, 37(11), 2825–2838. https://doi.org/10.1007/s10815-020-01929-w
Gong, D., Zhang, C., Li, T., Zhang, J., Zhang, N., Tao, Z., Zhu, W., & Sun, X. (2017). Are Sertoli cells a kind of mesenchymal stem cells? Am J Transl Res, 9(3), 1067–1074.
Yuan, Y., Li, L., Cheng, Q., Diao, F., Zeng, Q., Yang, X., Wu, Y., Zhang, H., Huang, M., Chen, J., Zhou, Q., Zhu, Y., Hua, R., Tian, J., Wang, X., Zhou, Z., Hao, J., Yu, J., Hua, D., … Sha, J. (2020). In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Research, 30(3), 244–255. https://doi.org/10.1038/s41422-020-0283-z
Aden, N. L., Bleeke, M., Kordes, U. R., Brunne, B., Holstermann, B., Biemann, R., Ceglarek, U., Soave, A., Salzbrunn, A., Schneider, S. W., & von Kopylow, K. (2023). Germ cell maintenance and sustained testosterone and precursor hormone production in human prepubertal testis organ culture with tissues from boys 7 years+ under conditions from adult testicular tissue. Cells, 12(3), 415. https://doi.org/10.3390/cells12030415
Wang, D., Hildorf, S., Ntemou, E., Mamsen, L. S., Dong, L., Pors, S. E., Fedder, J., Clasen-Linde, E., Cortes, D., Thorup, J., & Andersen, C. Y. (2022). Organotypic culture of testicular tissue from ınfant boys with cryptorchidism. International Journal of Molecular Sciences, 23(14), 7975. https://doi.org/10.3390/ijms23147975
Portela, J. M. D., de Winter-Korver, C. M., van Daalen, S. K. M., Meißner, A., de Melker, A. A., Repping, S., & van Pelt, A. M. M. (2019). Assessment of fresh and cryopreserved testicular tissues from (pre)pubertal boys during organ culture as a strategy for in vitro spermatogenesis. Human Reproduction, 34(12), 2443–2455. https://doi.org/10.1093/humrep/dez180
Sun, M., Yuan, Q., Niu, M., Wang, H., Wen, L., Yao, C., Hou, J., Chen, Z., Fu, H., Zhou, F., Li, C., Gao, S., Gao, W.-Q., Li, Z., & He, Z. (2018). Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death & Differentiation, 25(4), 749–766. https://doi.org/10.1038/s41418-017-0015-1
Mohammadzadeh, E., Mirzapour, T., Nowroozi, M. R., Nazarian, H., Piryaei, A., Alipour, F., Modarres Mousavi, S. M., & Ghaffari Novin, M. (2019). Differentiation of spermatogonial stem cells by soft agar three-dimensional culture system. Artificial Cells, Nanomedicine, and Biotechnology, 47(1), 1772–1781. https://doi.org/10.1080/21691401.2019.1575230
Yang, S., Hu, H., Kung, H., Zou, R., Dai, Y., Hu, Y., Wang, T., Lv, T., & Yu, J. (2020). Li F (2023) Organoids: The current status and biomedical applications. MedComm, 4(3), e274. https://doi.org/10.1002/mco2.274
Sakib, S., Uchida, A., Valenzuela-Leon, P., Yu, Y., Valli-Pulaski, H., Orwig, K., Ungrin, M., & Dobrinski, I. (2019). Formation of organotypic testicular organoids in microwell culture†. Biology of Reproduction, 100(6), 1648–1660. https://doi.org/10.1093/biolre/ioz053
Pendergraft, S. S., Sadri-Ardekani, H., Atala, A., & Bishop, C. E. (2017). Three-dimensional testicular organoid: A novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biology of Reproduction, 96(3), 720–732. https://doi.org/10.1095/biolreprod.116.143446
Dey, M., & Ozbolat, I. T. (2020). 3D bioprinting of cells, tissues and organs. Scientific Reports, 10(1), 14023. https://doi.org/10.1038/s41598-020-70086-y
Robinson, M., Bedford, E., Witherspoon, L., Willerth, S. M., & Flannigan, R. (2022). Using clinically derived human tissue to 3-dimensionally bioprint personalized testicular tubules for in vitro culturing: First report. F S Sci, 3(2), 130–139. https://doi.org/10.1016/j.xfss.2022.02.004
Tran, K. T. D., Valli-Pulaski, H., Colvin, A., & Orwig, K. E. (2022). Male fertility preservation and restoration strategies for patients undergoing gonadotoxic therapies†. Biology of Reproduction, 107(2), 382–405. https://doi.org/10.1093/biolre/ioac072
Önen, S., Gizer, M., Korkusuz, P. (2023). Flow cytometric and immunohistochemical follow-up of spermatogonial lineage commitment. Methods Mol Biol. https://doi.org/10.1007/7651_2023_506
Jabari, A., Gholami, K., Khadivi, F., Koruji, M., Amidi, F., Gilani, M. A. S., Mahabadi, V. P., Nikmahzar, A., Salem, M., Movassagh, S. A., Feizollahi, N., & Abbasi, M. (2023). In vitro complete differentiation of human spermatogonial stem cells to morphologic spermatozoa using a hybrid hydrogel of agarose and laminin. International Journal of Biological Macromolecules, 235, 123801. https://doi.org/10.1016/j.ijbiomac.2023.123801
Jabari, A., Sadighi Gilani, M. A., Koruji, M., Gholami, K., Mohsenzadeh, M., Rastegar, T., Khadivi, F., Ghanami Gashti, N., Nikmahzar, A., Mojaverrostami, S., Talebi, A., Ashouri Movassagh, S., Rezaie, M. J., & Abbasi, M. (2020). Three-dimensional co-culture of human spermatogonial stem cells with Sertoli cells in soft agar culture system supplemented by growth factors and Laminin. Acta Histochemica, 122(5), 151572. https://doi.org/10.1016/j.acthis.2020.151572
Khadivi, F., Koruji, M., Akbari, M., Jabari, A., Talebi, A., Ashouri Movassagh, S., Panahi Boroujeni, A., Feizollahi, N., Nikmahzar, A., Pourahmadi, M., & Abbasi, M. (2020). Application of platelet-rich plasma (PRP) improves self-renewal of human spermatogonial stem cells in two-dimensional and three-dimensional culture systems. Acta Histochemica, 122(8), 151627. https://doi.org/10.1016/j.acthis.2020.151627
de Michele, F., Poels, J., Vermeulen, M., Ambroise, J., Gruson, D., Guiot, Y., & Wyns, C. (2018). Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Frontiers in Physiology, 9, 1413. https://doi.org/10.3389/fphys.2018.01413
Medrano, J. V., Vilanova-Pérez, T., Fornés-Ferrer, V., Navarro-Gomezlechon, A., Martínez-Triguero, M. L., García, S., Gómez-Chacón, J., Povo, I., Pellicer, A., Andrés, M. M., & Novella-Maestre, E. (2018). Influence of temperature, serum, and gonadotropin supplementation in short- and long-term organotypic culture of human immature testicular tissue. Fertility and Sterility, 110(6), 1045-1057.e1043. https://doi.org/10.1016/j.fertnstert.2018.07.018
von Kopylow, K., Schulze, W., Salzbrunn, A., Schaks, M., Schäfer, E., Roth, B., Schlatt, S., & Spiess, A. N. (2018). Dynamics, ultrastructure and gene expression of human in vitro organized testis cells from testicular sperm extraction biopsies. Molecular Human Reproduction, 24(3), 123–134. https://doi.org/10.1093/molehr/gax070
Baert, Y., De Kock, J., Alves-Lopes, J. P., Söder, O., Stukenborg, J. B., & Goossens, E. (2017). Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Reports, 8(1), 30–38. https://doi.org/10.1016/j.stemcr.2016.11.012
de Michele, F., Poels, J., Weerens, L., Petit, C., Evrard, Z., Ambroise, J., Gruson, D., & Wyns, C. (2017). Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Human Reproduction, 32(1), 32–45. https://doi.org/10.1093/humrep/dew300
Rossant, J., & Tam, P. P. L. (2022). Early human embryonic development: Blastocyst formation to gastrulation. Developmental Cell, 57(2), 152–165. https://doi.org/10.1016/j.devcel.2021.12.022
Ilic, D., & Ogilvie, C. (2022). Pluripotent stem cells in clinical setting—new developments and overview of current status. Stem Cells, 40(9), 791–801. https://doi.org/10.1093/stmcls/sxac040
Baghbaderani, B. A., Syama, A., Sivapatham, R., Pei, Y., Mukherjee, O., Fellner, T., Zeng, X., & Rao, M. S. (2016). Detailed characterization of human induced pluripotent stem cells manufactured for therapeutic applications. Stem Cell Reviews and Reports, 12(4), 394–420. https://doi.org/10.1007/s12015-016-9662-8
Li, J., Wu, Y., Yao, X., Tian, Y., Sun, X., Liu, Z., Ye, X., & Wu, C. (2023). Preclinical research of stem cells: challenges and progress. Stem Cell Reviews and Reports, 19(6), 1676–1690. https://doi.org/10.1007/s12015-023-10528-y
Yamanaka, S. (2020). Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell, 27(4), 523–531. https://doi.org/10.1016/j.stem.2020.09.014
Higuchi, A., Ling, Q.-D., Kumar, S. S., Munusamy, M. A., Alarfaj, A. A., Chang, Y., Kao, S.-H., Lin, K.-C., Wang, H.-C., & Umezawa, A. (2015). Generation of pluripotent stem cells without the use of genetic material. Laboratory Investigation, 95(1), 26–42. https://doi.org/10.1038/labinvest.2014.132
Kurkure, P., Prasad, M., Dhamankar, V., & Bakshi, G. (2015). Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reproductive Biology and Endocrinology, 13, 1–9.
Haridhasapavalan, K. K., Borgohain, M. P., Dey, C., Saha, B., Narayan, G., Kumar, S., & Thummer, R. P. (2019). An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene, 686, 146–159. https://doi.org/10.1016/j.gene.2018.11.069
Romualdez-Tan, M. V. (2023). Modelling in vitro gametogenesis using induced pluripotent stem cells: A review. Cell Regeneration, 12(1), 33. https://doi.org/10.1186/s13619-023-00176-5
Hwang, Y. S., Suzuki, S., Seita, Y., Ito, J., Sakata, Y., Aso, H., Sato, K., Hermann, B. P., & Sasaki, K. (2020). Reconstitution of prospermatogonial specification in vitro from human induced pluripotent stem cells. Nature Communications, 11(1), 5656. https://doi.org/10.1038/s41467-020-19350-3
Lim, J. J., Shim, M. S., Lee, J. E., & Lee, D. R. (2014). Three-step method for proliferation and differentiation of human embryonic stem cell (hESC)-derived male germ cells. PLoS ONE, 9(4), e90454. https://doi.org/10.1371/journal.pone.0090454
Xu, H., Yang, M., Tian, R., Wang, Y., Liu, L., Zhu, Z., Yang, S., Yuan, Q., Niu, M., Yao, C., Zhi, E., Li, P., Zhou, C., He, Z., Li, Z., & Gao, W. Q. (2020). Derivation and propagation of spermatogonial stem cells from human pluripotent cells. Stem Cell Research & Therapy, 11(1), 408. https://doi.org/10.1186/s13287-020-01896-0
Acknowledgements
This review was written using the experience gained and research conducted within the scope of projects supported by The Scientific and Technological Research Council of Turkey (#120S254) and Hacettepe University Research Council (#TSA-2021-19204).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was funded by the The Scientific and Technological Research Council of Turkey (#120S254) and Hacettepe University Research Council (#TSA-2021–19204).
Author information
Authors and Affiliations
Contributions
M. G. and P. K. designed the study. M. G., S. Ö. and P. K. contributed to writing this review. M. G. and S. Ö. prepared figures. All authors participated of reviewing. P. K. supervised writing, editing, and reviewing of the article.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
Consent for publication was taken from all individuals.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gizer, M., Önen, S. & Korkusuz, P. The Evolutionary Route of in vitro Human Spermatogenesis: What is the Next Destination?. Stem Cell Rev and Rep 20, 1406–1419 (2024). https://doi.org/10.1007/s12015-024-10726-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-024-10726-2