Abstract
Ameloblasts are the specialized dental epithelial cell type responsible for enamel formation. Following completion of enamel development in humans, ameloblasts are lost and biological repair or regeneration of enamel is not possible. In the past, in vitro models to study dental epithelium and ameloblast biology were limited to freshly isolated primary cells or immortalized cell lines, both with limited translational potential. In recent years, large strides have been made with the development of induced pluripotent stem cell and organoid models of this essential dental lineage – both enabling modeling of human dental epithelium. Upon induction with several different signaling factors (such as transforming growth factor and bone morphogenetic proteins) these models display elevated expression of ameloblast markers and enamel matrix proteins. The advent of 3D bioprinting, and its potential combination with these advanced cellular tools, is poised to revolutionize the field – and its potential for tissue engineering, regenerative and personalized medicine. As the advancements in these technologies are rapidly evolving, we evaluate the current state-of-the-art regarding in vitro cell culture models of dental epithelium and ameloblast lineage with a particular focus toward their applicability for translational tissue engineering and regenerative/personalized medicine.
Graphical Abstract
Future perspectives for in vitro modeling of dental epithelium and ameloblasts. Development of iPSC and organoid models that can reliably generate dental epithelium and ameloblast-like cells, together with advances in 3D bioprinting, provide promising tools for enamel research. Advanced models will provide new avenues for development of enamel repair/regeneration approaches, for testing of dental materials or drugs, studying host-pathogen and/or cell-cell interactions, in vitro modeling of enamel diseases (e.g. amelogenesis imperfecta) and developing novel insights in fundamental tooth biology (e.g. regulation of amelogenesis, lineage specification). Abbreviations: iPSC: induced pluripotent stem cells; TO: tooth organoids; DE: dental epithelium; AB: ameloblast.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Reciprocal epithelial-mesenchymal interactions are the driving forces throughout the various stages of tooth development. Whereas the dental mesenchymal compartment contributes to most cells in mature tooth, including dental pulp and periodontal ligament, as well as to the dentin layer which is produced by odontoblasts, the dental epithelium eventually differentiates into ameloblasts (AB), the specialized cell type that forms enamel covering the crown of the tooth [1, 2].
The ameloblast lifecycle consists of four well-defined morphological and functional stages (Fig. 1). In the first step, inner enamel epithelium cells (presecretory ameloblasts; preAB) initiate cytodifferentiation by inducing differentiation of adjacent dental mesenchyme into odontoblasts, which deposit a fine layer of pre-dentin at the future dentinoenamel junction, in turn reciprocally instructing preAB to differentiate into secretory-stage AB (sAB) [3, 4]. During this process, preAB break through the basement membrane at the dentinoenamel junction, elongate from short cuboidal into tall columnar cells, and form so-called Tomes’ processes containing the cells’ secretory machinery at their apical enamel-forming ends. sAB secrete enamel matrix proteins such as amelogenin (AMELX), enamelin (ENAM) and ameloblastin (AMBN) to form a soft protein-rich enamel matrix, which is modified and stabilized by secreted proteases such as matrix metalloproteinase 20 (MMP20) [5, 6]. Using a variety of mineral and bicarbonate transporters, AB can drive mineral growth within the matrix, with each AB eventually forming a thin enamel rod or enamel crystallite [7]. In the third stage, AB transition from the secretory to the maturation stage by retracting their Tomes’ processes and depositing a new basal lamina. During this phase, and again during the final stage, approximately 25% of AB undergo apoptosis [8, 9]. Eventually, in the fourth stadium, maturation-stage AB (mAB) cycle between two morphologies at the enamel surface: ruffle-ended and smooth-ended, and enamel matrix protein expression shifts from predominantly AMELX and AMBN to the expression of odontogenic, AB associated (ODAM) and amelotin (AMTN) [10]. mAB degrade and reabsorb the enamel protein matrix through secretion of proteolytic enzymes (e.g. kallikrein-related peptidase-4 (KLK4)), while the enamel crystallites continue to grow and expand. Importantly, pH fluctuation has been found essential to the maturation phase, and pH levels appear closely intertwined with mAB morphology and function [11,12,13]. Acidification, which gradually increases during the ruffle-ended phase, is essential for proper maturation of hydroxyapatite crystallites. Once completed, the initially proteinaceous enamel matrix is highly mineralized with only little amounts of protein remaining. At the end of their lifecycle, all AB either contribute to the reduced enamel epithelium (a thin epithelial layer covering the enamel prior to eruption) or undergo apoptosis. After this phase, enamel is considered largely unable to be repaired or (re-)generated.
Schematic overview of the ameloblast lifecycle. Ameloblasts (AB) undergo a linear differentiation trajectory during amelogenesis consisting of four main stages, as defined by histological and functional properties: (1) pre-secretory stage, (2) secretory stage, (3) transitional stage and (4) maturation stage. Upon completion of amelogenesis (“post-maturation”), most AB undergo apoptosis, or contribute to reduced enamel epithelium (REE), causing the tooth to lose all enamel-reparative/regenerative capacity. Abbreviations: preAB: presecretory ameloblasts; sAB: secretory-stage ameloblasts; mAB: maturation-stage ameloblasts; BM: basement membrane; Ca2+: calcium; H+: hydrogen ion SV: secretory vesicles; EMP: enamel matrix proteins (e.g. AMELX, AMBN); MMP20: matrix metalloproteinase 20; KLK: kallikrein-related kinase 4; REE: reduced enamel epithelium
Each compartment may be separately affected in patients, as is the case in congenital disorders of tooth enamel and dentin (i.e. amelogenesis and dentinogenesis imperfecta, respectively; or as the enamel lesions caused by caries) [14, 15]. Alternatively, both dental mesenchyme- and epithelium-derived compartments may be jointly affected as for example in deep caries (which passes through enamel and dentin layers toward the pulp chamber) or in case of tooth loss or agenesis. To repair tooth defects in the field of restorative dentistry and tissue engineering, the strategy must therefore depend on the tooth compartment affected. Thus, to develop biological tooth repair and replacement strategies, reliable methods are required to first expand dental mesenchymal and epithelial compartments, and subsequently obtain differentiated dentin-producing odontoblasts and enamel-forming AB, respectively. Whereas considerable research efforts are aimed at developing tooth bioengineering and regenerative strategies, most studies start from the dental mesenchymal compartment (focusing on dental pulp and periodontal ligament) which alone will not be able to reconstitute the dental epithelial compartment and allow enamel regeneration [16]. Therefore, in this review, we evaluate the current state-of-the-art regarding in vitro cell culture models of dental epithelium, and their potential for expansion and AB production (Fig. 2; Table 1).
Overview of in vitro models of dental epithelium and ameloblasts. Firstly, primary AB (A) and IDECL (B) were the gold standard to study DE and AB in vitro. Recently, iPSC models (C) and 3D culture, either embedded in ECM (D, left), in suspension (D, right) or as organoids (E), have revolutionized the field. Abbreviations: AB: ameloblasts; IDECL: immortalized dental epithelial cell lines; iPSC: induced pluripotent stem cells; ECM: extracellular matrix; TO: tooth organoids
Main text
Primary Ameloblast Cells
Primary AB from rodent molars and incisors can be harvested and used for in vitro experimentation (Fig. 2A) [17,18,19,20,21,22,23]. Typically, mandibles are isolated, surrounding soft tissues are carefully excised, and the incisor enamel organ is micro-dissected. Using anatomical features, such as molar landmarks or morphological differences between stratum intermedium and papillary cells underlying respectively sAB or mAB, it is possible to delineate between secretory, transitional and maturation stages (for instance, see Houari et al. for video guidance on this procedure) [21, 24,25,26]. However, the use of spatial landmarks is not always straightforward, as positioning or tissue integrity might alter with age, or in pathological conditions [26]. Using enzymatic and mechanical digestion, distinct AB populations can be separately isolated and cultured in 2D, with serum-containing medium. A major drawback of this method is the possibility of contamination by other surrounding cells, such as stratum intermedium or connective tissue cells. Therefore, several studies have used fluorescent labeling to identify AB (e.g. using AMELX, AMBN) and/or exclude stromal cells (e.g. using CD90 as a marker) [18, 20]. Additionally, isolated AB must be used within 24-72 h of isolation, as these cells no longer proliferate and lack survival and expandability in vitro. Importantly, the same methodology can be employed to isolate AB fractions for RNA and protein analyses. Due to the difficulty maintaining primary AB in culture, many studies combine primary AB with other in vitro AB models (e.g. immortalized dental epithelial cell lines; see below).
Freshly isolated AB are most frequently used for in vitro study of the mechanisms and (dys-)regulation of ion metabolism (including ion channels and transporters, ameloblast-specific organelle biology) involved during enamel mineralization, for example using calcium imaging, (whole cell) patch clamping and ultrastructural imaging [18,19,20,21]. However, primary AB are employed in a wide array of study designs, for instance to evaluate the influence of environmental exposure (e.g. exposure to fluoride or bisphenol A and other endocrine disruptors) on different stages of amelogenesis, or to study developmental amelogenesis defects (e.g. in combination with mouse models of the developmental enamel disorder amelogenesis imperfecta) [22, 23, 27,28,29].
Although human primary dental epithelium/AB-like cell cultures starting from fetal tooth germ have been reported, their use is infrequent, and ethical concerns limit their use [30, 31]. Moreover, in contrast to primary AB from rodents, culture of fetal human AB-like cells was achieved by digestion and selective subculture of dental epithelial cells from fetal tooth germ, likely resulting in spontaneous immortalization (see further), and not all cells expressed AB-like features (e.g. expression of enamel matrix proteins). Therefore, primary rodent AB remain an essential in vitro tool for the tooth biologist to advance our understanding of fundamental AB and enamel biology. As new models are developed, their AB-like phenotype should be benchmarked against primary AB cells. Finally, due to their short-lived nature and the lack of human-derived primary AB, these cells are not usable for use in tissue engineering and regenerative medicine strategies.
Immortalized Dental Epithelial Cell Lines
Secondly, numerous immortalized dental epithelial cell lines (IDECL; recently reviewed by Zeng et al. (2023)) have been developed from the enamel organ of various species including mouse (e.g. ALC, LS8 and EOE-2 M/3 M), rat (e.g. HAT-7, SF2), pig (PABSo-E) and human (h-ALC) (Fig. 2B; Table 2) [31,32,33,34,35,36,37,38,39]. Similarly, other human immortalized cell lines were also derived from primary ameloblastoma (a benign tumor of the odontogenic epithelium), Hertwig’s Epithelial Root Sheath (HERS, a transient dental epithelial population crucial for root development), or of Epithelial Cell Rests of Malassez (ERM; located in the dental follicle and/or periodontal ligament) [40,41,42,43,44]. In general, immortalization of odontogenic tissues has been achieved using spontaneous immortalization, overexpression of viral oncogenes (e.g. SV40 or HP16 E6/E7 genes) or ectopic expression of human telomerase reverse transcriptase (hTERT) [32]. Genomic manipulation to produce immortalized cell lines has been associated with increased genomic instability (i.e. a state in which genomic mutations occur at higher frequency), including chromosomal instability (defined as a cellular state during in which unwarranted chromosomal changes in number and structure occurring at a high rate) [32, 45]. As a consequence IDECL may become unstable and heterogeneous (within and between different laboratories), resulting in skewed and unreproducible results. A community effort to characterize the genomic profiles of IDECL, and implement standardized genomic monitoring (e.g. karyotyping, comparative genome hybridization), would be a tremendous step forward. Nonetheless, these models recapitulate some key features of AB (e.g. expression of enamel matrix proteins, in vitro/vivo mineralization potential) making them a frequently used tool to probe processes involved in AB differentiation and develop novel 3D cell culture models (see further). Due to their ease of culture, IDECL are typically used to benchmark newly developed in vitro AB models – although IDECL are less physiologically relevant than isolated primary AB.
Interestingly, some IDECL have been reported to skew toward either preAB (SF2), sAB (LS8) or mAB (ALC) phenotype, which should be evaluated for other IDECL (Table 2) [46]. In addition, comparisons between different IDECL (e.g. to study molar- and incisor-specific differences in the dental epithelium) are challenging because most IDECL were developed using different methodologies, from different tooth types, at different developmental time points and from various species (Table 2). One could envision a collaborative effort to develop and make available IDECL using standardized methodologies and from corresponding developmental time points. In the meantime, researchers must exert caution and select the most appropriate IDECL for their study design. Thus, although IDECL can be expanded indefinitely (i.e. useful to obtain the required cell numbers for regenerative medicine strategies), presence of genomic instability and resulting genetic abnormalities further limits overall translatability and usefulness for regenerative medicine and tissue engineering endeavors.
Induced Pluripotent Stem Cells
A third method to model dental epithelium and AB is based on differentiation from induced pluripotent stem cells (iPSC) (and to a lesser extent on embryonic stem cells (ESC)) (Fig. 2C). iPSC are obtained by reprogramming somatic cells to dedifferentiate and acquire a pluripotent stem cell phenotype capable of self-renewal and differentiation into cells of all three germ layers. Although several methods exist, reprogramming is usually achieved by forced overexpression of specific transcription factors (TF) such as octamer-binding transcription factor 4 (Oct4), SRY (sex determining region Y)-box 2 (Sox2), Kruppel-like factor 4 (Klf4) and c-myc.
Overall, three fundamental approaches (either alone or in combination) have been applied to induce dental epithelium and/or AB differentiation from mouse and human iPSC. As a first strategy, through either co-culture with IDECL (as feeders instead of mouse embryonic fibroblasts) or application of conditioned medium from IDECL, iPSC are induced to acquire an epithelial phenotype and/or AB-like features (typically assessed by evaluation of enamel matrix protein expression) [36, 42, 47,48,49]. Alternatively, most recent protocols aim to replicate the sequential steps of AB development by mirroring key developmental cues (Fig. 3; Table 3) [50,51,52,53]. Typically, these procedures start by developing embryoid bodies from iPSC (i.e. spontaneously formed 3D aggregates comprising cells from all three germ layers upon suspension culture of iPSC), which are then guided toward an epithelial oral ectodermal fate, typically through stimulation of bone morphogenetic protein (BMP), retinoic acid (RA) and/or sonic hedgehog (SHH) signaling pathways. Next, dental epithelial fate is acquired, and cells can be pushed toward AB-like cells. Currently, no clear consensus protocol has been achieved, although modulation of BMP, SHH, Wingless-type MMTV integration site (WNT), epidermal growth factor (EGF) and transforming growth factor β (TGFβ) signaling appear crucial herein (Fig. 3; Table 3) [50,51,52,53]. Thirdly, through co-culture with dental mesenchymal cells (derived from embryonic day 14–16 molar tooth germs; see further), shown to possess tooth-inductive capabilities, and subsequent in vivo maturation (typically by transplantation under the kidney capsule), iPSC can differentiate into AB-like cells and produce mineralized tissues [48, 49, 53]. Although used as a protocol to induce differentiation in earlier studies, this methodology has shifted toward a validation tool, i.e. to confirm the in vivo differentiation potential of obtained differentiated cell products rather than the endpoint [50, 52, 53].
Overview of common signaling pathway modulation strategies for generation of ameloblast-like cells (based on Tables 3 and 4) in iPSC and tooth organoid models. Abbreviations: DE: dental epithelium; AB: ameloblast; RA: retinoic acid; ALK5: activin receptor-like kinase 5; SHH: sonic hedgehog; BMP: bone morphogenetic protein; NOG: Noggin; TGFβ: transforming growth factor β; SMAD: mothers against decapentaplegic; NT4: neurotrophin 4; EGF: epidermal growth factor; WNT: Wingless-type MMTV integration site
Even though iPSC are a potent tool with numerous advantages, such as low invasiveness of procurement, recapitulation of patient-specific genetic background, limitless supply of cells, and reduced risks of immune rejection when using a patient’s own cells, several aspects may affect their functionality for enamel and/or tooth tissue engineering. Firstly, with the aim of pursuing regenerative medicine, there are inherent risks associated with the use of oncogenes for reprogramming and the genetically unstable nature of reprogramming, which may promote tumor formation [54, 55]. However, use of non-integrating vectors may mitigate some of these risks [55,56,57]. Secondly, donor-specific genetic and epigenetic background may have undesired effects on the final product. It is well established that after reprogramming, iPSC still retain some epigenetic memory of their tissue of origin. Depending on the application, the presence of epigenetic memory can either impair or enhance lineage-specific differentiation of iPSC. For example, reprogrammed pancreatic β-cells were able to more robustly differentiate into insulin-producing β-cells compared to fibroblast-derived iPSC [58]. Several research groups have established iPSC lines from dental mesenchymal origins, such as from the dental pulp or periodontal ligament, whereas dental epithelium-derived iPSC have not yet been established [59,60,61]. To our knowledge, the effects of epigenetic memory have not yet been evaluated for iPSC in dental applications. Further study is required to develop dental epithelium-derived iPSC- (e.g. from ERM found in dental follicle or periodontal ligament, as done for tooth organoids; see below), and scrutinize the effects of epigenetic memory and iPSC origin (e.g. skin fibroblast-, dental mesenchyme- or epithelium-derived) on dental epithelium/AB differentiation efficacy and potential.
3D Cell Models of the Dental Epithelium
Non-Expandable 3D Culture of Immortalized Dental Epithelial Cell Lines and Dental Spheroids
In parallel with the development of IDECL and AB differentiation protocols for iPSC, researchers have been turning toward 3D culture of these cells in an attempt to further improve differentiation toward AB-like cells (Fig. 2D). Most simply, IDECL (ALC, SF2 or HAT-7) were expanded in a traditional 2D cell culture setting before seeding the cells (often together with fetal or postnatal dental mesenchymal cells) in an extracellular matrix (ECM), typically Matrigel (i.e. an ECM produced by a mouse sarcoma cell line and rich in basement membrane proteins such as laminin, collagen IV and entactin) (Fig. 2D) [62, 63]. Compared to their 2D counterparts, 3D-cultured IDECL typically showed enhanced expression of enamel matrix proteins such as AMELX. However, it was not evaluated whether these models could be expanded once seeded in the 3D environment.
Tadaki et al. (2016) were able to improve AB-like differentiation of immortalized rat SF2 cells by 3D suspension culture in fabricated polydimethylsiloxane scaffolds [64]. However, this system did not show any long-term expandability of cells once cultured in 3D. Also in 3D suspension culture, Tsunematsu et al. (2016) reported the formation of spheroids from immortalized human ERM. Although formed ERM-spheroids displayed stemness features, no expandability or dental epithelium/AB-like differentiation was reported [40]. Similarly, Natsiou et al. (2017) established the 3D culture of primary incisor labial cervical loop cells in Matrigel, displaying a keratinized stratified squamous epithelial phenotype. This study did not report any expression of AB markers, nor the possibility to expand these cultures [65]. In contrast, several years earlier, Chang et al. (2013) described a procedure for the generation of long-term expandable mouse dental epithelial stem cell spheres also established from the incisor labial cervical loop [66]. Although 3D-cultured spheres lacked expression of AB markers such as enamel matrix proteins, some enamel matrix protein expression could be induced by dissociating the spheres and seeding the cells in 2D together with a defined differentiation cocktail containing mineralization-inducing factors typically applied to stromal cells, such as dexamethasone, β-glycerophosphate, ascorbic acid and elevated calcium concentration.
Therefore, similarly to other dental epithelium model systems, 3D-cultured IDECL and dental spheroids still present important shortcomings to be used as a tool for tooth and/or enamel bioengineering strategies. Clear consensus protocols have not emerged and only Chang et al. (2013) showed the development of a long-term expandable culture of primary dental epithelial cells from mouse incisor, albeit lacking in AB-like differentiation potential [66].
Ex Vivo Organ Culture of Tooth Germs
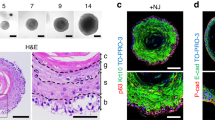
Ex vivo organ culture of embryonic tooth germs, typically followed by in vivo (e.g. subrenal) transplantation of the formed structures, has been a foundational staple in the field since the 70s [67, 68]. Since the early 2000s, researchers have been working on developing biological whole tooth replacement strategies using tooth germ culture approaches. In 2002, Young. et al. (2017) recombined dissociated primary dental epithelial and mesenchymal cells obtained from porcine tooth germs in a degradable polyglycolate/poly-L-lactate and poly-L-lactate-co-glycolate scaffold, which successfully formed mature tooth structures (including enamel, dentin and pulp) after 20–30 weeks of transplantation in rat omentum [69]. Similar results were obtained when both cell types from postnatal day 4 rat molar tooth germs (cap stage) were seeded on polyglycolate/poly-L-lactate and poly-L-lactate-co-glycolate scaffolds and transplanted into the rat omentum or a tooth extraction site [70, 71]. These developments were further improved by development of ‘bioengineered tooth germ’ technology (Fig. 4). Starting from embryonic day 14.5 mouse molar and incisor tooth germs (cap stage), dental epithelium and dental mesenchyme are separately dissociated into single cells, and subsequently recombined in collagen droplets [72]. Essentially, at this developmental stage, the dental mesenchyme possess tooth-inductive capacity, enabling development of mature dental epithelial tissue when recombined with other non-dental epithelial cell sources (e.g. human gingival epithelium, keratinocytes, iPSC) [48,49,50, 52, 73, 74]. Importantly, both cell types must be seeded at high density and compartmentalized within adjacent layers allowing direct cell-cell contact to enable success. Following ten days in vitro growth, transplantation under the subrenal capsule or in a tooth extraction site, tooth structures can be formed. Since its conception, this technology has been further enhanced and validated: bioengineered tooth germs can generate fully functioning and mature tooth when transplanted into a tooth extraction site, even becoming innervated and vascularized, and containing periodontal ligament [75, 76]. Moreover, using a size-control device, the shape and length of the bioengineered tooth germ can be regulated so that it is similar in size to a natural tooth [76]. Interestingly, in one bioengineered tooth germ multiple tooth primordia are frequently observed [72]. By developing a ligature-based method to split these primordia into individual tooth germs the yield could be enhanced [77]. Further, bioengineered tooth germ technology has also successfully been applied in beagles from both embryonic and postnatal tooth germs: at postnatal day 30 the tooth germs of permanent premolars were at a suitable stage (i.e. cap stage) to generate bioengineered tooth germ [78]. Importantly, this study also demonstrated autologous transplantation of postnatal canine bioengineered tooth germ, bringing the bioengineered tooth germ technology closer to the clinic.
Schematic overview of ‘bioengineered tooth germ’ technology. After collection of tooth germs from embryonal (typically day 14–16) mouse molar and incisor tooth germs, dental epithelium and dental mesenchyme are separately dissociated into single cells, and subsequently recombined at high cellular density in collagen droplets for ex vivo organ culture. Typically, organ cultures are transplanted into the kidney capsule of mice after 2–7 days of ex vivo culture to drive cytodifferentiation and formation of tooth structures
Unfortunately, application of bioengineered tooth germ technology is severely limited by the requirement of cap stage dental (mesenchymal) tissues, which are not easily collected from patients. Third molars or wisdom teeth are the last teeth to develop, are considered rudimentary and are routinely extracted, making them the most suitable candidate to collect tooth germs from. However, the average age of the initiation of third molar mineralization is between 7 and 10 years old, which would require tooth germs to be extracted before this point to develop bioengineered tooth germ [79,80,81]. Thus, the application of the bioengineered tooth germ technology for human biological tooth replacement requires further advances in obtaining postnatal dental stem cell sources, which can be guided toward a cap stage phenotype. Finally, as previously indicated, ex vivo recombination with murine tooth-inductive embryonal dental mesenchyme, and subsequent in vivo transplantation, are frequently used in conjunction with newly developed dental epithelium/AB models to validate their in vivo differentiation potential, which is essential for their further application in tooth bioengineering.
Tooth Organoids
In the last 15 years, the emergence of the organoid technology has drastically reshaped biomedical research by providing researchers the ability to mirror (parts of) organs and their development, phenotype and function in vitro [82,83,84]. Organoids are typically defined as self-forming and self-organizing 3D cell models that strongly mimic the physiology and sometimes morphology of their in vivo counterpart (i.e. much more than traditional 2D monolayer cell cultures). Establishment of organoids can start from iPSC/ESC, tissue-resident epithelial stem cells or stem cell-containing tissue fragments, and has been successful from many organs. In addition to their tissue epithelium-mirroring properties, organoids developed from epithelial tissue (stem) cells are highly and long-term expandable while genomically and transcriptionally stable, and retain their phenotypical and functional properties during the extensive culture time [83, 84]. Additionally, through optimization of the organoid medium (typically shifting from stemness-promoting medium components to known differentiation-inducing signals) or co-culture with other cell types, several organoid models may acquire a differentiated cell state [84]. In addition, organoid lines can be established from patients’ diseased tissue biopsies and mimic key aspects of the disease phenotype in vitro, making them powerful tools for disease modeling and for personalized medicine, either to determine the optimal therapeutic action or for use in regenerative medicine approaches [83,84,85,86]. The fact that organoids are highly and long-term expandable, genomically stable, as well as able to differentiate has strongly boosted the path to future regenerative therapies, for instance as recently shown for human bile ducts [85]. This landmark study showed for the first time the possibility to transplant human organoids into a live human organ (ex vivo), with organoids functionally engrafting in the host tissue. Thus, organoid technology is an exciting tool for many researchers strongly enabling both fundamental and applied research endeavors. Moreover, development of organoids from dental epithelial tissue would overcome many of the limitations associated with current options (Supplemental Appendix).
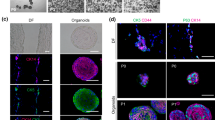
Recently, our group was the first to develop epithelial organoids starting from human and postnatal mouse tooth, followed by Kim et al. who derived incisor organoids from adult mouse (Fig. 2E; Table 4) [87,88,89]. Human tooth(-derived) organoids (TO) were established from the ERM obtained from the dental follicle of unerupted third molars [87]. Established human TO display a dental epithelial and stem cell phenotype similar to the ERM. Importantly, human TO are also able to mirror important biological functions of the ERM. Firstly, ERM are known to become proliferative and undergo EMT due to elevated EGF signals following infection, trauma, or orthodontic tooth movement [90]. This response to EGF is mimicked in vitro, when EGF-treated human TO become proliferative and undergo EMT. Secondly, human TO are amenable to both AB- and periodontal ligament-like differentiation in vitro. Developmentally, ERM are derived from HERS and have been ascribed to contribute to cementum and/or periodontal ligament repair and regeneration [91, 92]. Moreover, ERM are known to express enamel matrix proteins in vivo and in vitro cultured ERM were also shown to be able to acquire AB-like properties [87, 93, 94]. In addition, AB-like differentiation is also enhanced when co-cultured in the presence of dental pulp stem cells (DPSC) or when treated with TGFβ1 [87].
Mouse TO were established from early-postnatal (day 7) human-resembling molars and ever-growing incisors, and from 9-week-old adult mouse incisor [88, 89]. Both protocols rely on typical organoid medium components containing WNT pathway activation (WNT3A, RSPO1), BMP inhibition (NOGGIN), EGF (notably lacking from developed human TO medium), activin receptor-like kinase 5 (ALK5) inhibition (A83-01) as well as N-acetylcysteine, nicotinamide and fibroblast growth factor 10 (FGF10). The protocol developed by Kim et al. resulted in a less complex medium, also containing Notch signaling activator dibenzazepine, whereas the protocol established by our group also contained FGF2, FGF8, SHH, insulin growth factor 1 (IGF1) and cholera toxin (which activates adenylate cyclase and elevates intracellular cAMP levels).
In both cases, developed mouse TO contain abundant cytokeratin presence in the organoid cores, and closely mirror essential aspects of the dental epithelium (e.g. expression of dental epithelial stem cell markers SOX2 or TP63). Importantly, early-postnatal molar and incisor TO recapitulate tooth-type specific features (not yet evaluated for adult incisor TO). For instance, the transcription factor ISL LIM homeobox 1 (ISL1), which is essential for incisor dental epithelium but dispensable for molar development, is abundantly present in incisor-, but not molar TO [95]. Comparably to human TO, early postnatal molar and incisor TO also respond to exogenous EGF supplementation similarly to reported in vivo observations. Whereas EGF supplementation (also a key component for adult incisor TO) strongly improves incisor TO proliferation and induces precocious eruption of incisors when injected perinatally, no significant effects were reported for molar TO or eruption of molars [96, 97]. This faithful application is a powerful advantage compared to other currently available models and enables in vitro scrutiny of tooth-type specific biology.
Importantly, both studies reported unique protocols able to mirror distinct aspects of AB-like differentiation in vitro [88, 89]. Whereas suspension culture (which mirrors the loss of basement membrane between dental epithelium and dental mesenchyme during the transition from preAB to sAB) and SHH activation enable mirroring of the sAB phase (formation of crystals containing AMELX and AMBN), 3D culture in Matrigel with BMP and TGFβ activation predisposes toward the mAB stage (high ODAM/AMTN). Sequential combination of both approaches (i.e. a first differentiation phase in suspension, followed by a return to Matrigel) provides an exciting stepping stone toward full, in vitro recapitulation of amelogenesis in follow-up studies.
Taken together, these properties, together with the benefits of organoids over other available dental epithelial cell culture models, make tissue-derived TO a promising candidate for enamel and/or whole-tooth regeneration approaches as well as enamel disease modeling (Supplemental Appendix). Importantly, as previously mentioned, organoids or 3D spheroids can also be developed starting from iPSC/ESC instead of tissue-derived stem cells. Such models have recently also been successfully achieved for dental epithlium/AB-differentiated human iPSC, showing AB-like features and tooth-forming capability when combined with embryonic day 14.5 dental mesenchyme in vivo [50, 52, 53]. Recently, Alghadeer et al. successfully established 3D spheroids starting from iPSC-derived dental epithelial/AB-like cells [53]. Following a 16-day induction protocol, cells were transferred to ultra-low attachment plates and developed spheroids in suspension. Importantly, the developed ‘ameloblast spheroids’ displayed sAB-mirroring polarity, and were able to mimic polarity defects as seen in amelogenesis imperfecta. Joint implantation of ameloblast and DPSC spheroids in a mouse kidney capsule model resulted in secretion of enamel matrix proteins and formation of mineralized tissue. Because iPSC can be easily derived from skin fibroblasts, these models have the benefit of being developed without the need for tooth extraction (as opposed to human TO). Yet, as described above, the use of iPSCs is also associated with several disadvantages such as potential genetic alterations during reprogramming process, incomplete reprogramming and the epigenetic memory of source cells in addition to the risks associated with remnant undifferentiated iPSC (i.e. risk of tumor formation, off-target differentiation).
Bioprinting Dental Epithelium
Bioprinting of 3D tissue constructs is a relatively new and rapidly growing technology that leverages conventional 3D printing of viable living cells embedded in an extracellular matrix (ECM)-like bioink to generate functional biomimetic tissues. Combination of tight spatial control with reproducible and high throughput production makes 3D bioprinting a powerful player in the field of regenerative and personalized medicine. Although beyond the scope of this review, and thoroughly covered elsewhere, crucial aspects for consideration when developing bioprinted tissue constructs are: (1) the cell source, (2) differentiation state of printed cells, (3) bioink and (4) printing strategy [98, 99]. Although numerous groups have achieved remarkable results in dentistry research (reviewed by Obregon et al. (2015) and Ostrovidov et al. (2023)), only limited studies have achieved bioprinting of dental epithelial tissues [100,101,102,103].
As native dental epithelial/AB cells are lost by apoptosis once enamel is formed, alternative cell sources must be used when 3D bioprinting dental epithelial/AB. Mohabatpour et al. (2022) combined HAT-7 cells, i.e. IDCEL, with a newly developed two-component bioink comprising alginate and carboxymethyl chitosan (water-soluble chitosan-derivative with anti-microbial and mucoadhesive properties) to bioprint scaffolds for enamel tissue regeneration [103]. Following 3D bioprinting in alginate- carboxymethyl chitosan, HAT-7 cells displayed high viability (> 80%), ALP expression and gene expression of AB markers (e.g. Dentin sialophosphoprotein (DSPP), AMBN, ENAM and KLK4). Additionally, after 14 days, increased calcium and phosphorus content indicated initiation of mineralization. Taken together, these findings suggest suitability of such 3D scaffolds for differentiating dental epithelial cells toward AB-like cells and their potential for enamel bioengineering and repair/regeneration [103].
Tang et al. (2022) were able to 3D bioprint gelatin methacrylate (GelMA) constructs containing both primary rat dental papilla cells and HERS cells, derivates from the inner and outer enamel epithelium [102]. However, the main goal of this study was to promote essential epithelial-mesenchymal interactions during bone regeneration, rather than developing dental epithelial constructs. GelMA-encapsulated cells showed high viability (> 80%), proliferation, and migration. Notably, following tooth extraction, transplantation in the alveolar socket of Sprague-Dawley rats enhanced osteogenic differentiation (i.e. expression of osteogenic markers collagen type I, osteocalcin, Runt-related transcription factor 2 (RUNX2) and DSPP). De novo bone formation was enhanced in constructs containing both cell types.
Future studies aimed at applying 3D bioprinting for enamel repair/regeneration should commence from iPSC and/or organoid-derived dental epithelial material, given their reduced safety concerns. Indeed, bioprinting of advanced, highly mature iPSC/organoid-derived structures has been.
achieved. Both bioprinting of undifferentiated/uncommitted and differentiated (e.g. iPSC-derived cardiomyocytes, endothelial cells, oligodendrocyte precursors, neuronal precursors) have been successful to obtain mature tissue constructs [104,105,106,107]. Bioprinting can support generation of large-scale, highly reproducible organoids with enhanced maturation in a high throughput fashion, thereby overcoming some of the shortcomings of organoid technology [108]. Recently, bioprinting of centimeter-scale tissues was accomplished by Brassard et al. (2020), who applied a novel 3D bioprinting technique, the so-called bioprinting-assisted tissue emergence (BATE), using organoid-forming intestinal stem cells [109]. Notably, maturation and lumenization of bioprinted tissue constructs were achieved by co-deposition of intestinal mesenchymal cells. Together, high-throughput 3D bioprinting of reproducible, large-scale, mature tissues derived from iPSC and/or organoids holds tremendous potential to revolutionize drug discovery and regenerative/personalized medicine.
Conclusion
As has become clear, the available tools for in vitro modeling of dental epithelium and AB have tremendously advanced in recent years. The onset of expandable organoid and iPSC models that are able to generate dental epithelium and AB-like cells, together with continuous advances in 3D bioprinting, provide exciting avenues for future research (i.e. tooth development, lineage specification, disease modeling) and have unlocked the door for developing strategies for biological enamel repair and regeneration (see Graphical Abstract). Importantly, we believe these differing approaches (e.g. iPSC versus organoids) are highly complementary and can cross-fertilize, where insights from one model can be applied to advance the other. Moreover, combination of these dental epithelium/AB models with the rapid and exciting developments in dental mesenchymal models (e.g. via co-culture, co-bioprinting), as well as breakthroughs in identifying and modulating tooth field-forming and -inhibiting signals (e.g. USAG-1, also known as SOSTDC1) will be the necessary next steps forward in the field [1, 110, 111].
Data Availability
Not applicable.
References
Hermans, F., Hemeryck, L., Lambrichts, I., Bronckaers, A., & Vankelecom, H. (2021). Intertwined signaling pathways governing tooth development: A give-and-take between canonical wnt and shh. Frontiers in Cell and Developmental Biology, 9, 3389. https://doi.org/10.3389/fcell.2021.758203
Yu, T., & Klein, O. D. (2020). Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development, 147, dev184754. https://doi.org/10.1242/dev.184754
Bei, M. (2009). Molecular genetics of ameloblast cell lineage. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 312, 437–444. https://doi.org/10.1002/jez.b.21261
Bartlett, J. D. (2013). Dental Enamel Development: Proteinases and their Enamel Matrix substrates. International Scholarly Research Notices, 2013, 1–24. https://doi.org/10.1155/2013/684607
Bartlett, J. D., Ryu ok, H., Xue, J., Simmer, J. P., & Margolis, H. C. (1998). Enamelysin mRNA displays a developmental defined pattern of expression and encodes a protein which degrades amelogenin. Connective Tissue Research, 39, 101–109. https://doi.org/10.3109/03008209809023916
Fukae, M., Tanabe, T., Uchida, T., Lee, S. K., Ryu, O. H., Murakami, C., et al. (1998). Enamelysin (matrix metalloproteinase-20): Localization in the developing tooth and effects of pH and calcium on amelogenin hydrolysis. Journal of Dental Research, 77, 1580–1588. https://doi.org/10.1177/00220345980770080501
Lacruz, R. S., & Enamel (2017). Molecular identity of its transepithelial ion transport system. Cell Calcium, 65, 1–7. https://doi.org/10.1016/j.ceca.2017.03.006
Smith, C. E., & Warshawsky, H. (1977). Quantitative analysis of cell turnover in the enamel organ of the rat incisor. Evidence for ameloblast death immediately after enamel matrix secretion. Anatomical Record, 187, 63–97. https://doi.org/10.1002/ar.1091870106
Abramyan, J., Geetha-Loganathan, P., Šulcová, M., & Buchtová, M. (2021). Role of cell death in Cellular processes during odontogenesis. Frontiers in Cell and Developmental Biology, 9, 1554. https://doi.org/10.3389/fcell.2021.671475
Ganss, B., & Abbarin, N. (2014). Maturation and beyond: Proteins in the developmental continuum from enamel epithelium to junctional epithelium. Frontiers in Physiology, 5, 371. https://doi.org/10.3389/fphys.2014.00371
Damkier, H. H., Josephsen, K., Takano, Y., Zahn, D., Fejerskov, O., & Frische, S. (2014). Fluctuations in surface pH of maturing rat incisor enamel are a result of cycles of H+-secretion by ameloblasts and variations in enamel buffer characteristics. Bone, 60, 227–234. https://doi.org/10.1016/j.bone.2013.12.018
Josephsen, K., Takano, Y., Frische, S., Praetorius, J., Nielsen, S., Aoba, T., et al. (2010). Ion transporters in secretory and cyclically modulating ameloblasts: A new hypothesis for cellular control of preeruptive enamel maturation. American Journal of Physiology. Cell Physiology, 299, 1299–1307. https://doi.org/10.1152/ajpcell.00218.2010/
Bronckers, A. L. J. J. (2017). Ion Transport by ameloblasts during Amelogenesis. Journal of Dental Research, 96, 243–253. https://doi.org/10.1177/0022034516681768
Smith, C. E., Poulter, J. A., Antanaviciute, A., Kirkham, J., Brookes, S. J., Inglehearn, C. F., & Mighell, A. J. (2017). Amelogenesis imperfecta; genes, proteins, and pathways. Frontiers in Physiology, 8, 435. https://doi.org/10.3389/fphys.2017.00435
Peres, M. A., Macpherson, L. M. D., Weyant, R. J., Daly, B., Venturelli, R., Mathur, M. R., et al. (2019). Oral diseases: A global public health challenge. The Lancet, 394, 249–260. https://doi.org/10.1016/S0140-6736(19)31146-8/
Zhang, W., & Yelick, P. C. (2021). Tooth repair and regeneration: Potential of Dental Stem cells. Trends in Molecular Medicine, 27, 501–511. https://doi.org/10.1016/j.molmed.2021.02.005
Suzawa, T., Itoh, N., Takahashi, N., Katagiri, T., Morimura, N., Kobayashi, Y., et al. (2006). Establishment of primary cultures for mouse ameloblasts as a model of their lifetime. Biochemical and Biophysical Research Communications, 345, 1247–1253. https://doi.org/10.1016/j.bbrc.2006.04.122
Aulestia, F. J., Groeling, J., Bomfim, G. H. S., Costiniti, V., Manikandan, V., Chaloemtoem, A., et al. (2020). Fluoride exposure alters Ca 2+ signaling and mitochondrial function in enamel cells. Science Signaling, 13. https://doi.org/10.1126/scisignal.aay0086
Costiniti, V., Bomfim, G. H. S., Neginskaya, M., Son, G. Y., Mitaishvili, E., Giacomello, M., et al. (2022). Mitochondria modulate ameloblast Ca 2+ signaling. FASEB Journal, 36. https://doi.org/10.1096/fj.202100602r
Nurbaeva, M. K., Eckstein, M., Devotta, A., Saint-Jeannet, J. P., Yule, D. I., Hubbard, M. J., et al. (2018). Evidence that calcium entry into calcium-transporting dental enamel cells is regulated by cholecystokinin, acetylcholine and ATP. Frontiers in Physiology, 9, 801. https://doi.org/10.3389/fphys.2018.00801
Nurbaeva, M. K., Eckstein, M., Concepcion, A. R., Smith, C. E., Srikanth, S., Paine, M. L., et al. (2015). Dental enamel cells express functional SOCE channels. Scientific Reports, 5, https://doi.org/10.1038/srep15803
Wang, L., Zhu, Y., & Wang, D. (2016). High-dose fluoride induces apoptosis and inhibits Ameloblastin Secretion in primary rat ameloblast. Biological Trace Element Research, 174, 402–409. https://doi.org/10.1007/S12011-016-0738-8
Wang, L., Zhu, Y., & Wang, D. (2016). High-fluoride acitivates the FasL signalling pathway and leads to damage of ameloblast ultrastructure. Archives of Oral Biology, 71, 31–37. https://doi.org/10.1016/j.archoralbio.2016.06.024
Houari, S., Babajko, S., Loiodice, S., Berdal, A., & Jedeon, K. (2018). Micro-dissection of Enamel Organ from Mandibular Incisor of rats exposed to environmental toxicants. Journal of Visualized Experiments, 133, e57081. https://doi.org/10.3791/57081
Smith, C. E., & Nanci, A. (1989). A method for sampling the stages of amelogenesis on mandibular rat incisors using the molars as a reference for dissection. Anatomical Record, 225, 257–266. https://doi.org/10.1002/ar.1092250312
Bui, A. T., Lukashova, L., Verdelis, K., Vasquez, B., Bhogadi, L., Gabe, C. M., et al. (2023). Identification of stages of amelogenesis in the continuously growing mandiblular incisor of C57BL/6J male mice throughout life using molar teeth as landmarks. Frontiers in Physiology, 14, 1144712. https://doi.org/10.3389/fphys.2023.1144712
Jedeon, K., Houari, S., Loiodice, S., Thuy, T. T., Le Normand, M., Berdal, A., et al. (2016). Chronic exposure to Bisphenol A exacerbates Dental Fluorosis in growing rats. Journal of Bone and Mineral Research, 31, 1955–1966. https://doi.org/10.1002/jbmr.2879
Jedeon, K., De La Dure-Molla, M., Brookes, S. J., Loiodice, S., Marciano, C., Kirkham, J., et al. (2013). Enamel defects reflect perinatal exposure to Bisphenol A. American Journal of Pathology, 183, 118. https://doi.org/10.1016/j.ajpath.2013.04.004
Jedeon, K., Loiodice, S., Salhi, K., Le Normand, M., Houari, S., Chaloyard, J., et al. (2016). Androgen receptor involvement in rat amelogenesis: An additional way for endocrine-disrupting chemicals to affect Enamel Synthesis. Endocrinology, 157, 4287–4296. https://doi.org/10.1210/EN.2016-1342
Yan, Q., Zhang, Y., Li, W., & DenBesten, P. K. (2006). Differentiation of human ameloblast-lineage cells in vitro. European Journal of Oral Sciences, 114, 154–158. https://doi.org/10.1111/J.1600-0722.2006.00304.X
DenBesten, P. K., Machule, D., Zhang, Y., Yan, Q., & Li, W. (2005). Characterization of human primary enamel organ epithelial cells in vitro. Archives of Oral Biology, 50, 689–694. https://doi.org/10.1016/j.archoralbio.2004.12.008
Zeng, Y., Liu, L., Huang, D., & Song, D. (2023). Immortalized cell lines derived from dental/odontogenic tissue. Cell and Tissue Research, 393, 1–15. https://doi.org/10.1007/S00441-023-03767-5
DenBesten, P. K., Gao, C., Li, W., Mathews, C. H. E., & Gruenert, D. C. (1999). Development and characterization of an SV40 immortalized porcine ameloblast-like cell line. European Journal of Oral Sciences, 107, 276–281. https://doi.org/10.1046/j.0909-8836.1999.eos107407.X
Kawano, S., Morotomi, T., Toyono, T., Nakamura, N., Uchida, T., Ohishi, M., et al. (2002). Establishment of Dental Epithelial Cell line (HAT-7) and the cell differentiation dependent on Notch Signaling Pathway. Connective Tissue Research, 43, 409–412. https://doi.org/10.1080/03008200290000637
Chen, L. S., Couwenhoven, R. I., Hsu, D., Luo, W., & Snead, M. L. (1992). Maintenance of amelogenin gene expression by transformed epithelial cells of mouse enamel organ. Archives of Oral Biology, 37, 771–778. https://doi.org/10.1016/0003-9969(92)90110-T
Arakaki, M., Ishikawa, M., Nakamura, T., Iwamoto, T., Yamada, A., Fukumoto, E., et al. (2012). Role of epithelial-stem cell interactions during Dental Cell differentiation. Journal of Biological Chemistry, 287, 10590–10601. https://doi.org/10.1074/jbc.m111.285874
Nakata, A., Kameda, T., Nagai, H., Ikegami, K., Duan, Y., Terada, K., et al. (2003). Establishment and characterization of a spontaneously immortalized mouse ameloblast-lineage cell line. Biochemical and Biophysical Research Communications, 308, 834–839. https://doi.org/10.1016/S0006-291X(03)01467-0
MacDougall, M., Mamaeva, O., Lu, C., & Chen, S. (2019). Establishment and characterization of immortalized mouse ameloblast-like cell lines. Orthodontics and Craniofacial Research, 22(Suppl 1), 134–141. https://doi.org/10.1111/ocr.12313
Nakamura, T., De Vega, S., Fukumoto, S., Jimenez, L., Unda, F., & Yamada, Y. (2008). Transcription factor epiprofin is essential for tooth morphogenesis by regulating epithelial cell fate and tooth number. Journal of Biological Chemistry, 283, 4825–4833. https://doi.org/10.1074/jbc.m708388200
Tsunematsu, T., Fujiwara, N., Yoshida, M., Takayama, Y., Kujiraoka, S., Qi, G., et al. (2016). Human odontogenic epithelial cells derived from epithelial rests of Malassez possess stem cell properties. Laboratory Investigation, 96, 1063–1075. https://doi.org/10.1038/labinvest.2016.85
Nam, H., Kim, J. H., Kim, J. W., Seo, B. M., Park, J. C., Kim, J. W., et al. (2014). Establishment of Hertwig’s epithelial root sheath/epithelial rests of Malassez cell line from human periodontium. Molecules and Cells, 37, 562–567. https://doi.org/10.14348/molcells.2014.0161
Kim, G. H., Yang, J., Jeon, D. H., Kim, J. H., Chae, G. Y., Jang, M., et al. (2020). Differentiation and establishment of Dental Epithelial-Like Stem cells derived from human ESCs and iPSCs. International Journal of Molecular Sciences, 21, 4384. https://doi.org/10.3390/ijms21124384
Hatakeyama, S., Mizusawa, N., Tsutsumi, R., Yoshimoto, K., Mizuki, H., Yasumoto, S., et al. (2011). Establishment of human dental epithelial cell lines expressing ameloblastin and enamelin by transfection of hTERT and cdk4 cDNAs. Journal of Oral Pathology & Medicine, 40, 227–234. https://doi.org/10.1111/J.1600-0714.2010.00950.X
Harada, H., Mitsuyasu, T., Nakamura, N., Higuchi, Y., Toyoshima, K., Taniguchi, A., et al. (1998). Establishment of ameloblastoma cell line, AM-1. Journal of Oral Pathology & Medicine, 27, 207–212. https://doi.org/10.1111/J.1600-0714.1998.TB01943.X
He, Z., Wilson, A., Rich, F., Kenwright, D., Stevens, A., Low, Y. S., et al. (2023). Chromosomal instability and its effect on cell lines. Cancer Reports, 6, e1822. https://doi.org/10.1002/cnr2.1822
Sarkar, J., Simanian, E. J., Tuggy, S. Y., Bartlett, J. D., Snead, M. L., Sugiyama, T., et al. (2014). Comparison of two mouse ameloblast-like cell lines for enamel-specific gene expression. Frontiers in Physiology, 5, 277. https://doi.org/10.3389/fphys.2014.00277
Yoshida, K., Sato, J., Takai, R., Uehara, O., Kurashige, Y., Nishimura, M., et al. (2015). Differentiation of mouse iPS cells into ameloblast-like cells in cultures using medium conditioned by epithelial cell rests of Malassez and gelatin-coated dishes. Medical Molecular Morphology, 48, 138–145. https://doi.org/10.1007/S00795-014-0088-6
Liu, L., Liu, Y. F., Zhang, J., Duan, Y. Z., & Jin, Y. (2016). Ameloblasts serum-free conditioned medium: Bone morphogenic protein 4-induced odontogenic differentiation of mouse induced pluripotent stem cells. Journal of Tissue Engineering and Regenerative Medicine, 10, 466–474. https://doi.org/10.1002/term.1742
Kim, E. J., Yoon, K. S., Arakaki, M., Otsu, K., Fukumoto, S., Harada, H., et al. (2019). Effective differentiation of Induced Pluripotent stem cells into Dental cells. Developmental Dynamics, 248, 129–139. https://doi.org/10.1002/dvdy.24663
Kim, E. J., Mai, H. N., Lee, D. J., Kim, K. H., Lee, S. J., & Jung, H. S. (2021). Strategies for differentiation of hiPSCs into dental epithelial cell lineage. Cell and Tissue Research, 386, 415–421. https://doi.org/10.1007/S00441-021-03512-W
Miao, X., Niibe, K., Fu, Y., Zhang, M., Nattasit, P., Ohori-Morita, Y., et al. (2022). Epiprofin Transcriptional activation promotes ameloblast induction from mouse Induced Pluripotent stem cells via the BMP-Smad Signaling Axis. Frontiers in Bioengineering and Biotechnology, 10, 890882. https://doi.org/10.3389/fbioe.2022.890882
Kim, K. H., Kim, E. J., Kim, H. Y., Li, S., & Jung, H. S. (2023). Fabrication of functional ameloblasts from hiPSCs for dental application. Frontiers in Cell and Developmental Biology, 11, 1164811. https://doi.org/10.3389/fcell.2023.1164811
Alghadeer, A., Hanson-Drury, S., Patni, A. P., Ehnes, D. D., Zhao, Y. T., Li, Z., et al. (2023). Single-cell census of human tooth development enables generation of human enamel. Devel?opmental Cell, 58, 2163–2180e9. https://doi.org/10.1016/j.devcel.2023.07.013
Singh, V. K., Kalsan, M., Kumar, N., Saini, A., & Chandra, R. (2015). Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Frontiers in Cell and Developmental Biology, 3, https://doi.org/10.3389/fcell.2015.00002
Bulcha, J. T., Wang, Y., Ma, H., Tai, P. W. L., & Gao, G. (2021). Viral vector platforms within the gene therapy landscape. Signal Transduction and Targeted Therapy, 6, 53. https://doi.org/10.1038/s41392-021-00487-6
Chen, F., Qi, X., Zhang, R., Wu, Z. Y., Yan, C. E., Li, J., et al. (2017). Episomal lentiviral vectors confer erythropoietin expression in dividing cells. Plasmid, 90, 15–19. https://doi.org/10.1016/j.plasmid.2017.02.001
Gurumoorthy, N., Nordin, F., Tye, G. J., Wan Kamarul Zaman, W. S., & Ng, M. H. (2022). Non-integrating lentiviral vectors in clinical applications: A glance through. Biomedicines, 10, 107. https://doi.org/10.3390/biomedicines10010107
Efrat, S. (2021). Epigenetic memory: Lessons from iPS cells derived from human β cells. Frontiers in Endocrinology, 11, 1063. https://doi.org/10.3389/fendo.2020.614234
Oda, Y., Yoshimura, Y., Ohnishi, H., Tadokoro, M., Katsube, Y., Sasao, M., et al. (2010). Induction of pluripotent stem cells from human third molar mesenchymal stromal cells. Journal of Biological Chemistry, 285, 29270–29278. https://doi.org/10.1074/jbc.m109.055889
Wada, N., Wang, B., Lin, N. H., Laslett, A. L., Gronthos, S., & Bartold, P. M. (2011). Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. Journal of Periodontal Research, 46, 438–447. https://doi.org/10.1111/J.1600-0765.2011.01358.X
Kawano, E., Toriumi, T., Iguchi, S., Suzuki, D., Sato, S., & Honda, M. (2017). Induction of neural crest cells from human dental pulp-derived induced pluripotent stem cells. Biomedical Research, 38, 135–147. https://doi.org/10.2220/biomedres.38.135
Li, W., Machule, D., Gao, C., & DenBesten, P. K. (2006). Growth of ameloblast-lineage cells in a three-dimensional Matrigel environment. European Journal of Oral Sciences, 114, 159–163. https://doi.org/10.1111/J.1600-0722.2006.00308.X
Földes, A., Sang-Ngoen, T., Kádár, K., Rácz, R., Zsembery, Á., DenBesten, P., et al. (2021). Three-Dimensional Culture of Ameloblast-originated HAT-7 cells for functional modeling of defective tooth enamel formation. Frontiers in Pharmacology, 12, 1387. https://doi.org/10.3389/fphar.2021.682654
Tadaki, M., Anada, T., Shiwaku, Y., Nakamura, T., Nakamura, M., Kojima, M., et al. (2016). A 3D culture model study monitoring differentiation of dental epithelial cells into ameloblast-like cells. RSC Advances, 6, 62109–62118. https://doi.org/10.1039/C6RA04570G
Natsiou, D., Granchi, Z., Mitsiadis, T. A., & Jimenez-Rojo, L. (2017). Generation of spheres from Dental Epithelial Stem cells. Frontiers in Physiology, 8. https://doi.org/10.3389/fphys.2017.00007
Chang, J. Y. F., Wang, C., Jin, C., Yang, C., Huang, Y., Liu, J., et al. (2013). Self-renewal and multilineage differentiation of mouse dental epithelial stem cells. Stem Cell Reports, 11, 990–1002. https://doi.org/10.1016/j.scr.2013.06.008
Kollar, E. J., & Baird, G. R. (1970). Tissue interactions in embryonic mouse tooth germs. I. reorganization of the dental epithelium during tooth-germ reconstruction. Journal of Embryology and Experimental Morphology, 24, 159–171. https://doi.org/10.1242/dev.24.1.159
Kollar, E. J., & Baird, G. R. (1970). Tissue interactions in embryonic mouse tooth germs. II. The inductive role of the dental papilla. Journal of Embryology and Experimental Morphology, 24, 173–186. https://doi.org/10.1242/dev.24.1.173
Young, C. S., Terada, S., Vacanti, J. P., Honda, M., Bartlett, J. D., & Yelick, P. C. (2002). Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. Journal of Dental Research, 81, 695–700. https://doi.org/10.1177/154405910208101008
Duailibi, M. T., Duailibi, S. E., Young, C. S., Bartlett, J. D., Vacanti, J. P., & Yelick, P. C. (2004). Bioengineered teeth from cultured rat tooth bud cells. Journal of Dental Research, 83, 523–528. https://doi.org/10.1177/154405910408300703
Duailibi, S. E., Duailibi, M. T., Zhang, W., Asrican, R., Vacanti, J. P., & Yelick, P. C. (2008). Bioengineered dental tissues grown in the rat jaw. Journal of Dental Research, 87, 745–750. https://doi.org/10.1177/154405910808700811
Nakao, K., Morita, R., Saji, Y., Ishida, K., Tomita, Y., Ogawa, M., et al. (2007). The development of a bioengineered organ germ method. Nature Methods, 4, 227–230. https://doi.org/10.1038/nmeth1012
Angelova Volponi, A., Kawasaki, M., & Sharpe, P. T. (2013). Adult human gingival epithelial cells as a source for whole-tooth bioengineering. Journal of Dental Research, 92, 329–334. https://doi.org/10.1177/0022034513481041
Wang, B., Li, L., Du, S., Liu, C., Lin, X., Chen, Y. P., et al. (2010). Induction of human keratinocytes into enamel-secreting ameloblasts. Development Biology, 344, 799. https://doi.org/10.1016/j.ydbio.2010.05.511
Ikeda, E., Morita, R., Nakao, K., Ishida, K., Nakamura, T., Takano-Yamamoto, T., et al. (2009). Fully functional bioengineered tooth replacement as an organ replacement therapy. Proceedings of the National Academy of Sciences, 106, 13475–13480. https://doi.org/10.1073/pnas.0902944106
Oshima, M., Mizuno, M., Imamura, A., Ogawa, M., Yasukawa, M., Yamazaki, H., et al. (2011). Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One, 6, e21531. https://doi.org/10.1371/journal.pone.0021531
Yamamoto, N., Oshima, M., Tanaka, C., Ogawa, M., Nakajima, K., Ishida, K., et al. (2015). Functional tooth restoration utilising split germs through re-regionalisation of the tooth-forming field. Scientific Reports, 5, 18393. https://doi.org/10.1038/srep18393
Ono, M., Oshima, M., Ogawa, M., Sonoyama, W., Satoshi Hara, E., Oida, Y., et al. (2017). Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Scientific Reports, 7, 44522. https://doi.org/10.1038/srep44522
Sarnat, H., Kaffe, I., Porat, J., & Amir, E. (2003). Developmental stages of the third Molar in Israeli Children. Pediatric Dentistry, 25, 373–377.
Liversidge, H. M. (2009). Timing of human mandibular third molar formation. Annals of Human Biology, 35, 294–321. https://doi.org/10.1080/03014460801971445
Jung, Y. H., & Cho, B. H. (2014). Radiographic evaluation of third molar development in 6- to 24-year-olds. Imaging Science in Dentistry, 44, 185. https://doi.org/10.5624/isd.2014.44.3.185
Sato, T., Vries, R. G., Snippert, H. J., Van De Wetering, M., Barker, N., Stange, D. E., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 459, 262–265. https://doi.org/10.1038/nature07935
Clevers, H. (2016). Modeling Development and Disease with Organoids. Cell, 165, 1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
Artegiani, B., & Clevers, H. (2018). Use and application of 3D-organoid technology. Human Molecular Genetics, 27, R99–107. https://doi.org/10.3389/fcell.2021.671475
Sampaziotis, F., Muraro, D., Tysoe, O. C., Sawiak, S., Beach, T. E., Godfrey, E. M., et al. (2021). Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science, 371, 839–846. https://doi.org/10.1126/science.aaz6964
Berkers, G., van Mourik, P., Vonk, A. M., Kruisselbrink, E., Dekkers, J. F., de Winter-de Groot, K. M., et al. (2019). Rectal organoids enable personalized treatment of cystic fibrosis. Cell Reports, 26, 1701–1708e3. https://doi.org/10.1016/j.celrep.2019.01.068
Hemeryck, L., Hermans, F., Chappell, J., Kobayashi, H., Lambrechts, D., Lambrichts, I., et al. (2022). Organoids from human tooth showing epithelial stemness phenotype and differentiation potential. Cellular and Molecular Life Sciences, 79, 153. https://doi.org/10.1007/s00018-022-04183-8
Hermans, F., Hemeryck, L., Bueds, C., Torres Pereiro, M., Hasevoets, S., Kobayashi, H., et al. (2023). Organoids from mouse molar and incisor as new tools to study tooth-specific biology and development. Stem Cell Reports, 18, 1166–1181. https://doi.org/10.1016/j.stemcr.2023.03.011
Kim, H. Y., Cooley, V., Kim, E. J., Li, S., Lee, J. M., Sheyfer, D., et al. (2023). Adult dental epithelial stem cell-derived organoids deposit hydroxylapatite biomineral. International Journal of Oral Science, 15, 55. https://doi.org/10.1038/s41368-023-00257-w
Davis, E. M. (2018). A review of the epithelial cell rests of Malassez on the Bicentennial of their description. Journal of Veterinary Dentistry, 35, 290–298. https://doi.org/10.1177/0898756418811957
Xiong, J., Gronthos, S., & Bartold, P. M. (2013). Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of Periodontal Ligament tissues. Periodontology 2000, 63, 217–233. https://doi.org/10.1111/prd.12023
Huang, X., Bringas, P., Slavkin, H. C., & Chai, Y. (2009). Fate of HERS during tooth root development. Development Biology, 334, 22–30. https://doi.org/10.1016/j.ydbio.2009.06.034
Takahashi, K., Shimonishi, M., Wang, R., Watanabe, H., & Kikuchi, M. (2012). Epithelial-mesenchymal interactions induce enamel matrix proteins and proteases in the epithelial cells of the rests of Malassez in vitro. European Journal of Oral Sciences, 120, 475–483. https://doi.org/10.1111/J.1600-0722.2012.01002.X
Shinmura, Y., Tsuchiya, S., Hata, K. I., & Honda, M. J. (2008). Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells. Journal of Cellular Physiology, 217, 728–738. https://doi.org/10.1002/jcp.21546
Naveau, A., Zhang, B., Meng, B., Sutherland, M. T., Prochazkova, M., Wen, T., et al. (2017). Isl1 controls patterning and mineralization of enamel in the continuously renewing mouse incisor. Journal of Bone and Mineral Research, 32, 2219–2231. https://doi.org/10.1002/jbmr.3202
Rhodes, J. A., Fitzgibbon, D. H., Macchiarulo, P. A., & Murphy, R. A. (1987). Epidermal growth factor-induced precocious incisor eruption is associated with decreased tooth size. Development Biology, 121, 247–252. https://doi.org/10.1016/0012-1606(87)90156-4
Cielinski, M. J., Jolie, M., Wise, G. E., & Marks, S. C. (1995). The contrasting effects of colony-stimulating factor-1 and epidermal growth factor on tooth eruption in the rat. Connective Tissue Research, 32, 165–169. https://doi.org/10.3109/03008209509013720
Sabzevari, A., Rayat Pisheh, H., Ansari, M., & Salati, A. (2023). Progress in bioprinting technology for tissue regeneration. Journal of Artificial Organs. https://doi.org/10.1007/s10047-023-01394-z
Mandrycky, C., Wang, Z., Kim, K., & Kim, D. H. (2016). 3D bioprinting for engineering complex tissues. Biotechnology Advances, 34, 422–434. https://doi.org/10.1016/j.biotechadv.2015.12.011
Ostrovidov, S., Ramalingam, M., Bae, H., Orive, G., Fujie, T., Shi, X., et al. (2023). Bioprinting and biomaterials for dental alveolar tissue regeneration. Frontiers in Bioengineering and Biotechnology, 11, 991821. https://doi.org/10.3389/fbioe.2023.991821
Obregon, F., Vaquette, C., Ivanovski, S., Hutmacher, D. W., & Bertassoni, L. E. (2015). Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial tissue Engineering. Journal of Dental Research, 94, 143S–152S. https://doi.org/10.1177/0022034515588885
Tang, H., Bi, F., Chen, G., Zhang, S., Huang, Y., Chen, J., et al. (2022). 3D-bioprinted recombination structure of Hertwig’s epithelial Root Sheath cells and Dental Papilla cells for alveolar bone regeneration. International Journal of Bioprinting, 8, 512. https://doi.org/10.18063/ijb.V8I3.512
Mohabatpour, F., Duan, X., Yazdanpanah, Z., Tabil, X. L., Lobanova, L., Zhu, N., et al. (2022). Bioprinting of alginate-carboxymethyl chitosan scaffolds for enamel tissue engineering in vitro. Biofabrication, 15, 015022. https://doi.org/10.1088/1758-5090/acab35
Gu, Q., Tomaskovic-Crook, E., Wallace, G. G., & Crook, J. M. (2017). 3D Bioprinting Human Induced Pluripotent Stem Cell constructs for in situ cell proliferation and successive multilineage differentiation. Advanced Healthcare Materials, 6, 1700175. https://doi.org/10.1002/adhm.201700175
Gao, G., Lee, J. H., Jang, J., Lee, D. H., Kong, J. S., Kim, B. S., et al. (2017). Tissue Engineered Bio-blood-vessels constructed using a tissue-specific Bioink and 3D Coaxial Cell Printing technique: A novel therapy for ischemic disease. Advanced Functional Materials, 27, 1700798. https://doi.org/10.1002/adfm.201700798
Noor, N., Shapira, A., Edri, R., Gal, I., Wertheim, L., & Dvir, T. (2019). 3D Printing of Personalized Thick and Perfusable Cardiac patches and hearts. Advanced Science, 6, 1900344. https://doi.org/10.1002/advs.201900344
Joung, D., Truong, V., Neitzke, C. C., Guo, S. Z., Walsh, P. J., Monat, J. R., et al. (2018). 3D printed stem-cell derived neural progenitors generate spinal cord scaffolds. Advanced Functional Materials, 28, 1801850. https://doi.org/10.1002/adfm.201801850
Lawlor, K. T., Vanslambrouck, J. M., Higgins, J. W., Chambon, A., Bishard, K., Arndt, D., et al. (2021). Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nature Materials, 20, 260–271. https://doi.org/10.1038/s41563-020-00853-9
Brassard, J. A., Nikolaev, M., Hübscher, T., Hofer, M., & Lutolf, M. P. (2021). Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nature Materials, 20, 22–29. https://doi.org/10.1038/s41563-020-00803-5
Murashima-Suginami, A., Kiso, H., Tokita, Y., Mihara, E., Nambu, Y., Uozumi, R., et al. (2021). Anti-USAG-1 therapy for tooth regeneration through enhanced BMP signaling. Science Advances, 7, eabf1798. https://doi.org/10.1126/sciadv.abf1798
Ravi, V., Murashima-Suginami, A., Kiso, H., Tokita, Y., Huang, C. L., Bessho, K., et al. (2023). Advances in tooth agenesis and tooth regeneration. Regenerative Therapy, 22, 160–168. https://doi.org/10.1016/j.reth.2023.01.004
Ning, F., Guo, Y., Tang, J., Zhou, J., Zhang, H., Lu, W., et al. (2010). Differentiation of mouse embryonic stem cells into dental epithelial-like cells induced by ameloblasts serum-free conditioned medium. Biochemical and Biophysical Research Communications, 394, 342–347. https://doi.org/10.1016/j.bbrc.2010.03.007
Cai, J., Zhang, Y., Liu, P., Chen, S., Wu, X., Sun, Y., et al. (2013). Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regeneration, 2, 6. https://doi.org/10.1186/2045-9769-2-6
Mai, H. N., Kim, E. J., & Jung, H. S. (2021). Application of hiPSCs in tooth regeneration via cellular modulation. Journal of Oral Biosciences, 63, 225–231. https://doi.org/10.1016/J.JOB.2021.05.002
Miao, X., Niibe, K., Zhang, M., Liu, Z., Nattasit, P., Ohori-Morita, Y., et al. (2021). Stage-specific role of Amelx activation in Stepwise Ameloblast induction from Mouse Induced Pluripotent Stem cells. International Journal of Molecular Sciences, 22, 7195. https://doi.org/10.3390/ijms22137195
He, P., Zhang, Y., Kim, S. O., Radlanski, R. J., Butcher, K., Schneider, R. A., et al. (2010). Ameloblast differentiation in the human developing tooth: Effects of extracellular matrices. Matrix Biology : Journal of the International Society for Matrix Biology, 29, 411–419. https://doi.org/10.1016/j.matbio.2010.03.001
Hemeryck, L., Lambrichts, I., Bronckaers, A., & Vankelecom, H. (2022). Establishing organoids from human tooth as a powerful Tool toward mechanistic research and regenerative therapy. Journal of Visualized Experiments, 182, 63671. https://doi.org/10.3791/63671
Acknowledgements
We are grateful to all members of the Laboratory for Research in Ischemic Stroke, Stem Cells and Angiogenesis (LISSA, Hasselt University) and the Laboratory for Tissue Plasticity in Health and Disease (KU Leuven). Figures were created using Adobe Illustrator and BioRender.com.
Funding
This work was supported by grants from KU Leuven (Research Fund) and Fund for Scientific Research (FWO) Flanders. FH was supported by a FWO project grant (G061819FWO) and SH by a BOF UHasselt funding (BOF22KP12).
Author information
Authors and Affiliations
Contributions
FH collected the information, wrote the manuscript, and designed the figures. SH contributed to collection of information, co-wrote, and critically revised the manuscript. HV critically revised the manuscript. AB critically revised the manuscript and co-designed the figures. IL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing financial interests. HV, IL and AB have a patent on the tooth organoid medium (PCT/EP2023/054422).
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hermans, F., Hasevoets, S., Vankelecom, H. et al. From Pluripotent Stem Cells to Organoids and Bioprinting: Recent Advances in Dental Epithelium and Ameloblast Models to Study Tooth Biology and Regeneration. Stem Cell Rev and Rep 20, 1184–1199 (2024). https://doi.org/10.1007/s12015-024-10702-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-024-10702-w