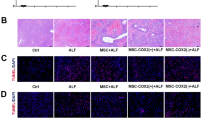
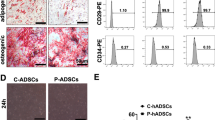
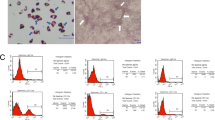
Abstract
Background
This study tested the hypothesis that inflammatory and interleukin (IL)-17 signalings were essential for acute liver ischemia (1 h)-reperfusion (72 h) injury (IRI) that was effectively ameliorated by adipose-derived mesenchymal stem cells (ADMSCs) and tacrolimus.
Methods
Adult-male SD rats (n = 50) were equally categorized into groups 1 (sham-operated-control), 2 (IRI), 3 [IRI + IL-17-monoclonic antibody (Ab)], 4 (IRI + tacrolimus), 5 (IRI + ADMSCs) and 6 (IRI + tacrolimus-ADMSCs) and liver was harvested at 72 h.
Results
The main findings included: (1) circulatory levels: inflammatory cells, immune cells, and proinflammatory cytokines as well as liver-damage enzyme at the time point of 72 h were highest in group 2, lowest in group 1 and significantly lower in group 6 than in groups 3 to 5 (all p < 0.0001), but they did not differ among these three latter groups; (2) histopathology: the liver injury score, fibrosis, inflammatory and immune cell infiltration in liver immunity displayed an identical pattern of inflammatory cells among the groups (all p < 0.0001); and (3) protein levels: upstream and downstream inflammatory signalings, oxidative-stress, apoptotic and mitochondrial-damaged biomarkers exhibited an identical pattern of inflammatory cells among the groups (all p < 0.0001).
Conclusion
Our results obtained from circulatory, pathology and molecular-cellular levels delineated that acute IRI was an intricate syndrome that elicited complex upstream and downstream inflammatory and immune signalings to damage liver parenchyma that greatly suppressed by combined tacrolimus and ADMSCs therapy.









Similar content being viewed by others
Data Availability
The data contained within the paper are available from the authors upon reasonable request.
Abbreviations
- IR:
-
ischemia and reperfusion
- IL:
-
interleukin
- ADMSCs:
-
adipose-derived mesenchymal stem cells
- MPO:
-
Myeloperoxidase
- CD:
-
cluster of differentiation
- TLR4:
-
Toll-like receptor 4
- TNF-α:
-
tumor nuclear factor-α
- HMGB1:
-
high-mobility group box 1
- TLR4:
-
Toll-like receptor 4
- TLR2:
-
Toll-like receptor 2
- MYD88:
-
myeloid differentiation primary response 88
- NF-κB:
-
nuclear factor-κB
- NOX-1:
-
NADPH oxidase 1
- NOX-2:
-
NADPH oxidase 2
- PARP:
-
Poly ADP-ribose Polymerase
- γ-H2AX:
-
γ-Histone H2AX
- WBC:
-
white blood cell
- ALT:
-
alanine aminotransferase
References
Khashab, M., Tector, A. J., & Kwo, P. Y. (2007). Epidemiology of acute liver failure. Current Gastroenterology Reports, 9(1), 66–73. https://doi.org/10.1007/s11894-008-0023-x.
Henrion, J. (2012). Hypoxic hepatitis. Liver International : Official Journal of the International Association for the Study of the Liver, 32(7), 1039–1052. https://doi.org/10.1111/j.1478-3231.2011.02655.x.
Fuhrmann, V., Kneidinger, N., Herkner, H., Heinz, G., Nikfardjam, M., Bojic, A., et al. (2009). Hypoxic hepatitis: Underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Medicine, 35(8), 1397–1405. https://doi.org/10.1007/s00134-009-1508-2.
Zhang, X. J., Cheng, X., Yan, Z. Z., Fang, J., Wang, X., Wang, W., et al. (2018). An ALOX12-12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nature Medicine, 24(1), 73–83. https://doi.org/10.1038/nm.4451.
Ito, T., Naini, B. V., Markovic, D., Aziz, A., Younan, S., Lu, M., et al. (2021). Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. American Journal of Transplantation, 21(2), 614–625. https://doi.org/10.1111/ajt.16219.
Leithead, J. A., Armstrong, M. J., Corbett, C., Andrew, M., Kothari, C., Gunson, B. K., et al. (2013). Hepatic ischemia reperfusion injury is associated with acute kidney injury following donation after brain death liver transplantation. Transplant International, 26(11), 1116–1125. https://doi.org/10.1111/tri.12175.
Klein, M., Geoghegan, J., Wangemann, R., Bockler, D., Schmidt, K., & Scheele, J. (1999). Preconditioning of donor livers with prostaglandin I2 before retrieval decreases hepatocellular ischemia-reperfusion injury. Transplantation, 67(8), 1128–1132. https://doi.org/10.1097/00007890-199904270-00007.
Bogetti, D., Sankary, H. N., Jarzembowski, T. M., Manzelli, A., Knight, P. S., Thielke, J., et al. (2005). Thymoglobulin induction protects liver allografts from ischemia/reperfusion injury. Clinical Transplantation, 19(4), 507–511. https://doi.org/10.1111/j.1399-0012.2005.00375.x.
Luntz, S. P., Unnebrink, K., Seibert-Grafe, M., Bunzendahl, H., Kraus, T. W., Buchler, M. W., et al. (2005). HEGPOL: Randomized, placebo controlled, multicenter, double-blind clinical trial to investigate hepatoprotective effects of glycine in the postoperative phase of liver transplantation [ISRCTN69350312]. Bmc Surgery, 5, 18. https://doi.org/10.1186/1471-2482-5-18.
Busuttil, R. W., Lipshutz, G. S., Kupiec-Weglinski, J. W., Ponthieux, S., Gjertson, D. W., Cheadle, C., et al. (2011). rPSGL-Ig for improvement of early liver allograft function: A double-blind, placebo-controlled, single-center phase II study. American Journal of Transplantation, 11(4), 786–797. https://doi.org/10.1111/j.1600-6143.2011.03441.x.
Lee, W. M. (2012). Acute liver failure. Seminars in Respiratory and Critical Care Medicine, 33(1), 36–45. https://doi.org/10.1055/s-0032-1301733.
Singanayagam, A., & Bernal, W. (2015). Update on acute liver failure. Current Opinion in Critical Care, 21(2), 134–141. https://doi.org/10.1097/MCC.0000000000000187.
Willars, C. (2014). Update in intensive care medicine: Acute liver failure. Initial management, supportive treatment and who to transplant. Current Opinion in Critical Care, 20(2), 202–209. https://doi.org/10.1097/MCC.0000000000000073.
de Rougemont, O., Lehmann, K., & Clavien, P. A. (2009). Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transplantation, 15(10), 1172–1182. https://doi.org/10.1002/lt.21876.
Jaeschke, H., & Woolbright, B. L. (2012). Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplantation Reviews (Orlando, Fla.), 26(2), 103–114. https://doi.org/10.1016/j.trre.2011.10.006.
Lv, X., Yang, L., Tao, K., Liu, Y., Yang, T., Chen, G., et al. (2011). Isoflurane preconditioning at clinically relevant doses induce protective effects of heme oxygenase-1 on hepatic ischemia reperfusion in rats. Bmc Gastroenterology, 11, 31. https://doi.org/10.1186/1471-230X-11-31.
Hu, C., Zhao, L., Zhang, F., & Li, L. (2021). Melatonin and its protective role in attenuating warm or cold hepatic ischaemia/reperfusion injury. Cell Proliferation, 54(4), e13021. https://doi.org/10.1111/cpr.13021.
Li, Y., Yang, Y., Feng, Y., Yan, J., Fan, C., Jiang, S., et al. (2014). A review of melatonin in hepatic ischemia/reperfusion injury and clinical liver disease. Annals of Medicine, 46(7), 503–511. https://doi.org/10.3109/07853890.2014.934275.
Lu, L., Zhou, H., Ni, M., Wang, X., Busuttil, R., Kupiec-Weglinski, J., et al. (2016). Innate Immune Regulations and Liver Ischemia-Reperfusion Injury. Transplantation, 100(12), 2601–2610. https://doi.org/10.1097/TP.0000000000001411.
Tomiyama, K., Ikeda, A., Ueki, S., Nakao, A., Stolz, D. B., Koike, Y., et al. (2008). Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology, 48(5), 1608–1620. https://doi.org/10.1002/hep.22482.
Abu-Amara, M., Yang, S. Y., Tapuria, N., Fuller, B., Davidson, B., & Seifalian, A. (2010). Liver ischemia/reperfusion injury: Processes in inflammatory networks–a review. Liver Transplantation, 16(9), 1016–1032. https://doi.org/10.1002/lt.22117.
Bhogal, R. H., Curbishley, S. M., Weston, C. J., Adams, D. H., & Afford, S. C. (2010). Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transplantation, 16(11), 1303–1313. https://doi.org/10.1002/lt.22157.
Uchida, Y., Freitas, M. C., Zhao, D., Busuttil, R. W., & Kupiec-Weglinski, J. W. (2010). The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation, 89(9), 1050–1056. https://doi.org/10.1097/TP.0b013e3181d45a98.
Klune, J. R., & Tsung, A. (2010). Molecular biology of liver ischemia/reperfusion injury: Established mechanisms and recent advancements. Surgical Clinics of North America, 90(4), 665–677. https://doi.org/10.1016/j.suc.2010.04.003.
Sun, C. K., Zhang, X. Y., Sheard, P. W., Mabuchi, A., & Wheatley, A. M. (2005). Change in mitochondrial membrane potential is the key mechanism in early warm hepatic ischemia-reperfusion injury. Microvascular Research, 70(1–2), 102–110. https://doi.org/10.1016/j.mvr.2005.04.003.
Sun, C. K., Zhang, X. Y., Zimmermann, A., Davis, G., & Wheatley, A. M. (2001). Effect of ischemia-reperfusion injury on the microcirculation of the steatotic liver of the Zucker rat. Transplantation, 72(10), 1625–1631. https://doi.org/10.1097/00007890-200111270-00008.
Besnard, A. G., Togbe, D., Couillin, I., Tan, Z., Zheng, S. G., Erard, F., et al. (2012). Inflammasome-IL-1-Th17 response in allergic lung inflammation. Journal of Molecular Cell Biology, 4(1), 3–10. https://doi.org/10.1093/jmcb/mjr042.
Llacuna, L., Mari, M., Lluis, J. M., Garcia-Ruiz, C., Fernandez-Checa, J. C., & Morales, A. (2009). Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappab inactivation in prolonged ischemia/reperfusion. American Journal of Pathology, 174(5), 1776–1785. https://doi.org/10.2353/ajpath.2009.080857.
Bettelli, E., Korn, T., Oukka, M., & Kuchroo, V. K. (2008). Induction and effector functions of T(H)17 cells. Nature, 453(7198), 1051–1057. https://doi.org/10.1038/nature07036.
Kono, H., Fujii, H., Ogiku, M., Hosomura, N., Amemiya, H., Tsuchiya, M., et al. (2011). Role of IL-17A in neutrophil recruitment and hepatic injury after warm ischemia-reperfusion mice. The Journal of Immunology, 187(9), 4818–4825. https://doi.org/10.4049/jimmunol.1100490.
Yang, X., Li, C., Ng, K. T., Liu, J., Liu, H., Zhang, W., et al. (2020). IL-17a exacerbates hepatic ischemia-reperfusion injury in fatty liver by promoting neutrophil infiltration and mitochondria-driven apoptosis. Journal of Leukocyte Biology, 108(5), 1603–1613. https://doi.org/10.1002/JLB.3MA0520-716R.
Sun, C. K., Leu, S., Hsu, S. Y., Zhen, Y. Y., Chang, L. T., Tsai, C. Y., et al. (2015). Mixed serum-deprived and normal adipose-derived mesenchymal stem cells against acute lung ischemia-reperfusion injury in rats. Am J Transl Res, 7(2), 209–231.
Chang, C. L., Sung, P. H., Sun, C. K., Chen, C. H., Chiang, H. J., Huang, T. H., et al. (2015). Protective effect of melatonin-supported adipose-derived mesenchymal stem cells against small bowel ischemia-reperfusion injury in rat. Journal of Pineal Research, 59(2), 206–220. https://doi.org/10.1111/jpi.12251.
Ko, S. F., Yip, H. K., Zhen, Y. Y., Lee, C. C., Lee, C. C., Huang, C. C., et al. (2015). Adipose-derived mesenchymal stem cell exosomes suppress Hepatocellular Carcinoma Growth in a rat model: Apparent diffusion coefficient, natural killer T-Cell responses, and histopathological features. Stem Cells Int, 2015, 853506. https://doi.org/10.1155/2015/853506.
Lin, K. C., Yip, H. K., Shao, P. L., Wu, S. C., Chen, K. H., Chen, Y. T., et al. (2016). Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. International Journal of Cardiology, 216, 173–185. https://doi.org/10.1016/j.ijcard.2016.04.061.
Chen, K. H., Chen, C. H., Wallace, C. G., Yuen, C. M., Kao, G. S., Chen, Y. L., et al. (2016). Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget, 7(46), 74537–74556. https://doi.org/10.18632/oncotarget.12902.
Chang, C. L., Leu, S., Sung, H. C., Zhen, Y. Y., Cho, C. L., Chen, A., et al. (2012). Impact of apoptotic adipose-derived mesenchymal stem cells on attenuating organ damage and reducing mortality in rat sepsis syndrome induced by cecal puncture and ligation. J Transl Med, 10, 244. https://doi.org/10.1186/1479-5876-10-244.
Chen, H. H., Lin, K. C., Wallace, C. G., Chen, Y. T., Yang, C. C., Leu, S., et al. (2014). Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. Journal of Pineal Research, 57(1), 16–32. https://doi.org/10.1111/jpi.12140.
Sung, P. H., Chiang, H. J., Chen, C. H., Chen, Y. L., Huang, T. H., Zhen, Y. Y., et al. (2016). Combined Therapy with adipose-derived mesenchymal stem cells and ciprofloxacin against Acute Urogenital Organ damage in Rat Sepsis Syndrome Induced by Intrapelvic injection of cecal Bacteria. Stem Cells Transl Med, 5(6), 782–792. https://doi.org/10.5966/sctm.2015-0116.
Milosavljevic, N., Gazdic, M., Simovic Markovic, B., Arsenijevic, A., Nurkovic, J., Dolicanin, Z., et al. (2018). Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells - an experimental study. Transplant International, 31(1), 102–115. https://doi.org/10.1111/tri.13023.
Timmerman, L. A., Clipstone, N. A., Ho, S. N., Northrop, J. P., & Crabtree, G. R. (1996). Rapid shuttling of NF-AT in discrimination of Ca2 + signals and immunosuppression. Nature, 383(6603), 837–840. https://doi.org/10.1038/383837a0.
Liu, J., Farmer, J. D. Jr., Lane, W. S., Friedman, J., Weissman, I., & Schreiber, S. L. (1991). Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell, 66(4), 807–815. https://doi.org/10.1016/0092-8674(91)90124-h.
Watanabe, S., Zhang, Y., Fukusumi, Y., Yasuda, H., Takada, A., Kazama, J. J., et al. (2022). Th17 cells participate in Thy1.1 Glomerulonephritis which is ameliorated by Tacrolimus. American Journal of Nephrology, 53(5), 388–396. https://doi.org/10.1159/000524111.
Jurewicz, W. A. (2003). Tacrolimus versus cyclosporin immunosuppression: Long-term outcome in renal transplantation. Nephrology, Dialysis, Transplantation, 18(Suppl 1), i7–11. https://doi.org/10.1093/ndt/gfg1028.
Chua, S., Leu, S., Sheu, J. J., Lin, Y. C., Chang, L. T., Kao, Y. H., et al. (2012). Intra-coronary administration of tacrolimus markedly attenuates infarct size and preserves heart function in porcine myocardial infarction. Journal of Inflammation (London), 9(1), 21. https://doi.org/10.1186/1476-9255-9-21.
Yang, C. H., Sheu, J. J., Tsai, T. H., Chua, S., Chang, L. T., Chang, H. W., et al. (2013). Effect of tacrolimus on myocardial infarction is associated with inflammation, ROS, MAP kinase and akt pathways in mini-pigs. J Atheroscler Thromb, 20(1), 9–22. https://doi.org/10.5551/jat.14316.
Sheu, J. J., Sung, P. H., Leu, S., Chai, H. T., Zhen, Y. Y., Chen, Y. C., et al. (2013). Innate immune response after acute myocardial infarction and pharmacomodulatory action of tacrolimus in reducing infarct size and preserving myocardial integrity. Journal of Biomedical Science, 20(1), 82. https://doi.org/10.1186/1423-0127-20-82.
Yang, C. C., Sung, P. H., Chiang, J. Y., Chai, H. T., Chen, C. H., Chu, Y. C., et al. (2021). Combined tacrolimus and melatonin effectively protected kidney against acute ischemia-reperfusion injury. The Faseb Journal, 35(6), e21661. https://doi.org/10.1096/fj.202100174R.
Sun, C. K., Chang, C. L., Lin, Y. C., Kao, Y. H., Chang, L. T., Yen, C. H., et al. (2012). Systemic administration of autologous adipose-derived mesenchymal stem cells alleviates hepatic ischemia-reperfusion injury in rats. Critical Care Medicine, 40(4), 1279–1290. https://doi.org/10.1097/CCM.0b013e31823dae23.
Ko, S. F., Yip, H. K., Leu, S., Lee, C. C., Sheu, J. J., Lee, C. C., et al. (2014). Therapeutic potential of tacrolimus on acute myocardial infarction in minipigs: Analysis with serial cardiac magnetic resonance and changes at histological and protein levels. Biomed Research International, 2014, 524078. https://doi.org/10.1155/2014/524078.
Yuen, C. M., Yeh, K. H., Wallace, C. G., Chen, K. H., Lin, H. S., Sung, P. H., et al. (2017). EPO-cyclosporine combination therapy reduced brain infarct area in rat after acute ischemic stroke: Role of innate immune-inflammatory response, micro-RNAs and MAPK family signaling pathway. Am J Transl Res, 9(4), 1651–1666.
Li, Y. C., Chen, C. H., Chang, C. L., Chiang, J. Y., Chu, C. H., Chen, H. H., et al. (2021). Melatonin and hyperbaric oxygen therapies suppress colorectal carcinogenesis through pleiotropic effects and multifaceted mechanisms. International Journal of Biological Sciences, 17(14), 3728–3744. https://doi.org/10.7150/ijbs.62280.
Li, Y. C., Sung, P. H., Yang, Y. H., Chiang, J. Y., Yip, H. K., & Yang, C. C. (2021). Dipeptidyl peptidase 4 promotes peritoneal fibrosis and its inhibitions prevent failure of peritoneal dialysis. Commun Biol, 4(1), 144. https://doi.org/10.1038/s42003-021-01652-x.
Gaffen, S. L., Kramer, J. M., Yu, J. J., & Shen, F. (2006). The IL-17 cytokine family. Vitamins and Hormones, 74, 255–282. https://doi.org/10.1016/S0083-6729(06)74010-9.
Maxwell, J. R., Zhang, Y., Brown, W. A., Smith, C. L., Byrne, F. R., Fiorino, M., et al. (2015). Differential Roles for Interleukin-23 and Interleukin-17 in intestinal immunoregulation. Immunity, 43(4), 739–750. https://doi.org/10.1016/j.immuni.2015.08.019.
Yang, X. O., Chang, S. H., Park, H., Nurieva, R., Shah, B., Acero, L., et al. (2008). Regulation of inflammatory responses by IL-17F. Journal of Experimental Medicine, 205(5), 1063–1075. https://doi.org/10.1084/jem.20071978.
Park, H., Li, Z., Yang, X. O., Chang, S. H., Nurieva, R., Wang, Y. H., et al. (2005). A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology, 6(11), 1133–1141. https://doi.org/10.1038/ni1261.
Hurst, S. D., Muchamuel, T., Gorman, D. M., Gilbert, J. M., Clifford, T., Kwan, S., et al. (2002). New IL-17 family members promote Th1 or Th2 responses in the lung: In vivo function of the novel cytokine IL-25. The Journal of Immunology, 169(1), 443–453. https://doi.org/10.4049/jimmunol.169.1.443.
Caldwell, C. C., Okaya, T., Martignoni, A., Husted, T., Schuster, R., & Lentsch, A. B. (2005). Divergent functions of CD4 + T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. American Journal of Physiology. Gastrointestinal and Liver Physiology, 289(5), G969–G976. https://doi.org/10.1152/ajpgi.00223.2005.
Mills, K. H. G. (2023). IL-17 and IL-17-producing cells in protection versus pathology. Nature Reviews Immunology, 23(1), 38–54. https://doi.org/10.1038/s41577-022-00746-9.
Harrington, L. E., Hatton, R. D., Mangan, P. R., Turner, H., Murphy, T. L., Murphy, K. M., et al. (2005). Interleukin 17-producing CD4 + effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology, 6(11), 1123–1132. https://doi.org/10.1038/ni1254.
Aggarwal, S., Ghilardi, N., Xie, M. H., de Sauvage, F. J., & Gurney, A. L. (2003). Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. Journal of Biological Chemistry, 278(3), 1910–1914. https://doi.org/10.1074/jbc.M207577200.
Iwakura, Y., & Ishigame, H. (2006). The IL-23/IL-17 axis in inflammation. J Clin Invest, 116(5), 1218–1222. https://doi.org/10.1172/JCI28508.
Ko, S. F., Sung, P. H., Yang, C. C., Chiang, J. Y., & Yip, H. K. (2022). Combined therapy with dapagliflozin and entresto offers an additional benefit on improving the heart function in rat after ischemia-reperfusion injury. Biomed J. https://doi.org/10.1016/j.bj.2022.06.002.
Chang, C. L., Chen, H. H., Chen, K. H., Chiang, J. Y., Li, Y. C., Lin, H. S., et al. (2019). Adipose-derived mesenchymal stem cell-derived exosomes markedly protected the brain against sepsis syndrome induced injury in rat. Am J Transl Res, 11(7), 3955–3971.
Acknowledgements
Not applicable.
Funding
This study was supported by a project grant from Chang Gung Memorial Hospital, Chang Gung University [CMRPG8L0791]. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
SFK, and HKY supervised the study and wrote the manuscript. SFK and YLC applied the grant project. SFK, YLC and PLS performed the experiments. YCL, JYC, PHS and YLC collected and analyzed data. YCL reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All animal procedures were approved by the Institute of Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital (Affidavit of Approval of Animal Use Protocol No. 2020092301) on 10/26/2020 and performed in accordance with the Guide for the Care and Use of Laboratory Animals. The approved title was “Interleukin-17 signaling pathway plays a crucial role on participating in acute liver ischemia-reperfusion injury—impact of immune-pharmaco-modulation of ADMSCs and tacrolimus.“
Consent for Publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi-Ling Chen and Hon-Kan Yip contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ko, SF., Li, YC., Shao, PL. et al. Interplay Between Inflammatory-immune and Interleukin-17 Signalings Plays a Cardinal Role on Liver Ischemia-reperfusion Injury—Synergic Effect of IL-17Ab, Tacrolimus and ADMSCs on Rescuing the Liver Damage. Stem Cell Rev and Rep 19, 2852–2868 (2023). https://doi.org/10.1007/s12015-023-10611-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10611-4