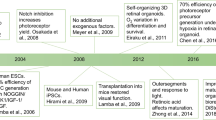
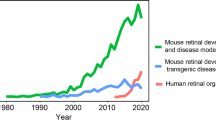
Abstract
The intricate neural circuit of retina extracts salient features of the natural world and forms bioelectric impulse as the origin of vision. The early development of retina is a highly complex and coordinated process in morphogenesis and neurogenesis. Increasing evidence indicates that stem cells derived human retinal organoids (hROs) in vitro faithfully recapitulates the embryonic developmental process of human retina no matter in the transcriptome, cellular biology and histomorphology. The emergence of hROs greatly deepens on the understanding of early development of human retina. Here, we reviewed the events of early retinal development both in animal embryos and hROs studies, which mainly comprises the formation of optic vesicle and optic cup shape, differentiation of retinal ganglion cells (RGCs), photoreceptor cells (PRs) and its supportive retinal pigment epithelium cells (RPE). We also discussed the classic and frontier molecular pathways up to date to decipher the underlying mechanisms of early development of human retina and hROs. Finally, we summarized the application prospect, challenges and cutting-edge techniques of hROs for uncovering the principles and mechanisms of retinal development and related developmental disorder. hROs is a priori selection for studying human retinal development and function and may be a fundamental tool for unlocking the unknown insight into retinal development and disease.
Graphical Abstract





Similar content being viewed by others
Data Availability
Not applicable.
References
Quinn, P. M. J., & Wijnholds, J. (2019). Retinogenesis of the human fetal retina: An apical polarity perspective. Genes, 10(12), 987. https://doi.org/10.3390/genes10120987
O’Hara-Wright, M., & Gonzalez-Cordero, A. (2020). Retinal organoids: a window into human retinal development. Development, 147(24). https://doi.org/10.1242/dev.189746
Wagstaff, P. E., Berzal, A. H., Boon, C. J. F., Quinn, P. M. J., ten Asbroek, A. L. M. A., & Bergen, A. A. (2021). The role of small molecules and their effect on the molecular mechanisms of early retinal organoid development. International Journal of Molecular Sciences, 22(13), 7081. https://doi.org/10.3390/ijms22137081
Chirco, K. R., Chew, S., Moore, A. T., Duncan, J. L., & Lamba, D. A. (2021). Allele-specific gene editing to rescue dominant CRX-associated LCA7 phenotypes in a retinal organoid model. Stem Cell Reports, 16(11), 2690–2702. https://doi.org/10.1016/j.stemcr.2021.09.007
Roger, J. E., & Goureau, O. (2022). Modeling PRPF31 retinitis pigmentosa using retinal pigment epithelium and organoids combined with gene augmentation rescue. Npj Regenerative Medicine, 7(1). https://doi.org/10.1038/s41536-022-00235-6
Li, M., Gong, J., Ge, L., Gao, H., Yang, J., Yang, C., Kang, J., Fang, Y., & Xu, H. (2022). Development of human retinal organoid models for bisphenol toxicity assessment. Ecotoxicology and Environmental Safety, 245, 114094. https://doi.org/10.1016/j.ecoenv.2022.114094
Zeng, Y., Li, M., Zou, T., Chen, X., Li, Q., Li, Y., Ge, L., Chen, S., & Xu, H. (2021). The Impact of Particulate Matter (PM2.5) on Human Retinal Development in hESC-Derived Retinal Organoids. Frontiers in Cell and Developmental Biology, 9. https://doi.org/10.3389/fcell.2021.607341
Rowan, S., Chen, C.-M.A., Young, T. L., Fisher, D. E., & Cepko, C. L. (2004). Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development, 131(20), 5139–5152. https://doi.org/10.1242/dev.01300
Heisenberg, C.-P., Houart, C., Take-uchi, M., Rauch, G.-J., Young, N., Coutinho, P., Masai, I., Caneparo, L., Concha, M. L., Geisler, R., Dale, T. C., Wilson, S. W., & Stemple, D. L. (2001). A mutation in the Gsk3–binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes & Development, 15(11), 1427–1434. https://doi.org/10.1101/gad.194301
Zuber, M. E., Gestri, G., Viczian, A. S., Barsacchi, G., & Harris, W. A. (2003). Specification of the vertebrate eye by a network of eye field transcription factors. Development, 130(21), 5155–5167. https://doi.org/10.1242/dev.00723
Gao, Z., & Godbout, R. (2011). Serine phosphorylation regulates disabled-1 early isoform turnover independently of Reelin. Cellular Signalling, 23(3), 555–565. https://doi.org/10.1016/j.cellsig.2010.11.007
Hendrickson, A. (2016). Development of retinal layers in prenatal human retina. American Journal of Ophthalmology, 161, 29-35.e1. https://doi.org/10.1016/j.ajo.2015.09.023
Duan, X., et al. (2020). Single-cell analysis of human retina identifies evolutionarily conserved and species-specific mechanisms controlling development. Developmental Cell, 53(4), 473-491.e9. https://doi.org/10.1016/j.devcel.2020.04.009
Jeon, S., & Oh, I.-H. (2015). Regeneration of the retina: Toward stem cell therapy for degenerative retinal diseases. BMB Reports, 48(4), 193–199. https://doi.org/10.5483/bmbrep.2015.48.4.276
Trapani, I., & Auricchio, A. (2018). Seeing the light after 25 years of retinal gene therapy. Trends in Molecular Medicine, 24(8), 669–681. https://doi.org/10.1016/j.molmed.2018.06.006
Sluch, V. M., Davis, C. O., Ranganathan, V., Kerr, J. M., Krick, K., Martin, R., Berlinicke, C. A., Marsh-Armstrong, N., Diamond, J. S., Mao, H.-Q., & Zack, D. J. (2015). Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Scientific Reports, 5(1). https://doi.org/10.1038/srep16595
Sluch, V. M., Chamling, X., Liu, M. M., Berlinicke, C. A., Cheng, J., Mitchell, K. L., Welsbie, D. S., & Zack, D. J. (2017). Enhanced stem cell differentiation and immunopurification of genome engineered human retinal ganglion cells. Stem Cells Translational Medicine, 6(11), 1972–1986. https://doi.org/10.1002/sctm.17-0059
Hirami, Y., Osakada, F., Takahashi, K., Okita, K., Yamanaka, S., Ikeda, H., Yoshimura, N., & Takahashi, M. (2009). Generation of retinal cells from mouse and human induced pluripotent stem cells. Neuroscience Letters, 458(3), 126–131. https://doi.org/10.1016/j.neulet.2009.04.035
Osakada, F., Ikeda, H., Mandai, M., Wataya, T., Watanabe, K., Yoshimura, N., Akaike, A., Sasai, Y., & Takahashi, M. (2008). Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nature Biotechnology, 26(2), 215–224. https://doi.org/10.1038/nbt1384
Xiao, D., Deng, Q., Guo, Y., Huang, X., Zou, M., Zhong, J., Rao, P., Xu, Z., Liu, Y., Hu, Y., Shen, Y., Jin, K., & Xiang, M. (2020). Generation of self-organized sensory ganglion organoids and retinal ganglion cells from fibroblasts. Science Advances, 6(22). https://doi.org/10.1126/sciadv.aaz5858
Hayashi, R., Ishikawa, Y., Sasamoto, Y., Katori, R., Nomura, N., Ichikawa, T., Araki, S., Soma, T., Kawasaki, S., Sekiguchi, K., Quantock, A. J., Tsujikawa, M., & Nishida, K. (2016). Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature, 531(7594), 376–380. https://doi.org/10.1038/nature17000
Tanaka, T., Yokoi, T., Tamalu, F., Watanabe, S.-I., Nishina, S., & Azuma, N. (2015). Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Scientific Reports, 5(1). https://doi.org/10.1038/srep08344
Nakano, T., Ando, S., Takata, N., Kawada, M., Muguruma, K., Sekiguchi, K., Saito, K., Yonemura, S., Eiraku, M., & Sasai, Y. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell, 10(6), 771–785. https://doi.org/10.1016/j.stem.2012.05.009
Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., Sekiguchi, K., Adachi, T., & Sasai, Y. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature, 472(7341), 51–56. https://doi.org/10.1038/nature09941
Cuevas, E., Holder, D. L., Alshehri, A. H., Tréguier, J., Lakowski, J., & Sowden, J. C. (2021). NRL −/− gene edited human embryonic stem cells generate rod-deficient retinal organoids enriched in S-cone-like photoreceptors. Stem Cells, 39(4), 414–428. https://doi.org/10.1002/stem.3325
Kallman, A., Capowski, E. E., Wang, J., Kaushik, A. M., Jansen, A. D., Edwards, K. L., Chen, L., Berlinicke, C. A., Phillips, M. J., Pierce, E. A., Qian, J., Wang, T.-H., Gamm, D. M., & Zack, D. J. (2020b). Investigating cone photoreceptor development using patient-derived NRL null retinal organoids. Communications Biology, 3(1). https://doi.org/10.1038/s42003-020-0808-5
Zou, T., Gao, L., Zeng, Y., Li, Q., Li, Y., Chen, S., Hu, X., Chen, X., Fu, C., Xu, H., & Yin, Z. Q. (2019a). Organoid-derived C-Kit+/SSEA4− human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-08961-0
Bian, B., Zhao, C., He, X., Gong, Y., Ren, C., Ge, L., Zeng, Y., Li, Q., Chen, M., Weng, C., He, J., Fang, Y., Xu, H., & Yin, Z. Q. (2020). Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. Journal of Extracellular Vesicles, 9(1), 1748931. https://doi.org/10.1080/20013078.2020.1748931
Gong, Y., He, X., Li, Q., He, J., Bian, B., Li, Y., Ge, L., Zeng, Y., Xu, H., & Yin, Z. Q. (2019). SCF/SCFR signaling plays an important role in the early morphogenesis and neurogenesis of human embryonic neural retina. Development. https://doi.org/10.1242/dev.174409
Kuwahara, A., Ozone, C., Nakano, T., Saito, K., Eiraku, M., & Sasai, Y. (2015a). Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nature Communications, 6(1). https://doi.org/10.1038/ncomms7286.
Singh, R. K., Winkler, P. A., Binette, F., Petersen-Jones, S. M., & Nasonkin, I. O. (2021). Comparison of developmental dynamics in human fetal retina and human pluripotent stem cell-derived retinal tissue. Stem Cells and Development, 30(8), 399–417. https://doi.org/10.1089/scd.2020.0085
Saha, A., Capowski, E., Zepeda, M. A. F., Nelson, E. C., Gamm, D. M., & Sinha, R. (2022). Cone photoreceptors in human stem cell-derived retinal organoids demonstrate intrinsic light responses that mimic those of primate fovea. Cell Stem Cell, 29(3), 460-471.e3. https://doi.org/10.1016/j.stem.2022.01.002
Zhong, X., Gutierrez, C., Xue, T., Hampton, C., Vergara, M. N., Cao, L.-H., Peters, A., Park, T. S., Zambidis, E. T., Meyer, J. S., Gamm, D. M., Yau, K.-W., & Canto-Soler, M. V. (2014). Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications, 5(1). https://doi.org/10.1038/ncomms5047
Norrie, J. L., Nityanandam, A., Lai, K., Chen, X., Wilson, M., Stewart, E., Griffiths, L., Jin, H., Wu, G., Orr, B., Tran, Q., Allen, S., Reilly, C., Zhou, X., Zhang, J., Newman, K., Johnson, D., Brennan, R., & Dyer, M. A. (2021a). Retinoblastoma from human stem cell-derived retinal organoids. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-24781-7
Gao, M.-L., Zhang, X., Han, F., Xu, J., Yu, S.-J., Jin, K., & Jin, Z.-B. (2022). Functional microglia derived from human pluripotent stem cells empower retinal organ. Science China Life Sciences. https://doi.org/10.1007/s11427-021-2086-0
Cakir, B., Xiang, Y., Tanaka, Y., Kural, M. H., Parent, M., Kang, Y.-J., Chapeton, K., Patterson, B., Yuan, Y., He, C.-S., Raredon, M. S. B., Dengelegi, J., Kim, K.-Y., Sun, P., Zhong, M., Lee, S., Patra, P., Hyder, F., Niklason, L. E., & Park, I.-H. (2019). Engineering of human brain organoids with a functional vascular-like system. Nature Methods, 16(11), 1169–1175. https://doi.org/10.1038/s41592-019-0586-5
Shi, Y., Sun, L., Wang, M., Liu, J., Zhong, S., Li, R., Li, P., Guo, L., Fang, A., Chen, R., Ge, W.-P., Wu, Q., & Wang, X. (2020). Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLOS Biology, 18(5), e3000705. https://doi.org/10.1371/journal.pbio.3000705
Fligor, C. M., Lavekar, S. S., Harkin, J., Shields, P. K., VanderWall, K. B., Huang, K.-C., Gomes, C., & Meyer, J. S. (2021). Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Reports, 16(9), 2228–2241. https://doi.org/10.1016/j.stemcr.2021.05.009
Achberger, K., Probst, C., Haderspeck, J., Bolz, S., Rogal, J., Chuchuy, J., Nikolova, M., Cora, V., Antkowiak, L., Haq, W., Shen, N., Schenke-Layland, K., Ueffing, M., Liebau, S., & Loskill, P. (2019a). Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. ELife, 8. https://doi.org/10.7554/elife.46188
Kenyon, K. L., Zaghloul, N., & Moody, S. A. (2001). 1–s2.0-S0012160601904646-main. Developmental Biology. https://doi.org/10.1006/dbio.2001.0464
Horsford, D. J., Nguyen, M.-T.T., Sellar, G. C., Kothary, R., Arnheiter, H., & McInnes, R. R. (2005). Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development, 132(1), 177–187. https://doi.org/10.1242/dev.01571
Bumsted, K. M., & Barnstable, C. J. (2000). Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi_mi) mouse. Investigative Ophthalmology & Visual Science
Nguyen-Ba-Charvet, K. T., & Rebsam, A. (2020). Neurogenesis and Specification of Retinal Ganglion Cells. International Journal of Molecular Sciences, 21(2), 451. https://doi.org/10.3390/ijms21020451
Gueta, K., David, A., Cohen, T., Menuchin-Lasowski, Y., Nobel, H., Narkis, G., Li, L., Love, P., de Melo, J., Blackshaw, S., Westphal, H., & Ashery-Padan, R. (2016). The stage-dependent roles of Ldb1 and functional redundancy with Ldb2 in mammalian retinogenesis. Development. https://doi.org/10.1242/dev.129734
Reichman, S., Terray, A., Slembrouck, A., Nanteau, C., Orieux, G., Habeler, W., Nandrot, E. F., Sahel, J.-A., Monville, C., & Goureau, O. (2014). From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proceedings of the National Academy of Sciences, 111(23), 8518–8523. https://doi.org/10.1073/pnas.1324212111
Hill, J. C. G., Davidson, D. R., & Robert E. (1995). Development, 1433
Smith, A. N., Miller, L.-A., Radice, G., Ashery-Padan, R., & Lang, R. A. (2009). Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development, 136(19), 3377–3377. https://doi.org/10.1242/dev.043802
Mellough, C. B., Collin, J., Khazim, M., White, K., Sernagor, E., Steel, D. H. W., & Lako, M. (2015). IGF-1 signaling plays an important role in the formation of three-dimensional laminated neural retina and other ocular structures from human embryonic stem cells. Stem Cells, 33(8), 2416–2430. https://doi.org/10.1002/stem.2023
Phillips, M. J., Perez, E. T., Martin, J. M., Reshel, S. T., Wallace, K. A., Capowski, E. E., Singh, R., Wright, L. S., Clark, E. M., Barney, P. M., Stewart, R., Dickerson, S. J., Miller, M. J., Percin, E. F., Thomson, J. A., & Gamm, D. M. (2014). Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells, 32(6), 1480–1492. https://doi.org/10.1002/stem.1667
Gamm, D. M., Clark, E., Capowski, E. E., & Singh, R. (2019). The role of FGF9 in the production of neural retina and RPE in a pluripotent stem cell model of early human retinal development. American Journal of Ophthalmology, 206, 113–131. https://doi.org/10.1016/j.ajo.2019.04.033
Capowski, E. E., Simonett, J. M., Clark, E. M., Wright, L. S., Howden, S. E., Wallace, K. A., Petelinsek, A. M., Pinilla, I., Phillips, M. J., Meyer, J. S., Schneider, B. L., Thomson, J. A., & Gamm, D. M. (2014). Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation. Human Molecular Genetics, 23(23), 6332–6344. https://doi.org/10.1093/hmg/ddu351
Nguyen, M., & Arnheiter, H. (2000). Signaling and transcriptional regulation in early mammalian eye development: A link between FGF and MITF. Development, 127(16), 3581–3591. https://doi.org/10.1242/dev.127.16.3581
Meyer, J. S., Shearer, R. L., Capowski, E. E., Wright, L. S., Wallace, K. A., McMillan, E. L., Zhang, S.-C., & Gamm, D. M. (2009). Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proceedings of the National Academy of Sciences, 106(39), 16698–16703. https://doi.org/10.1073/pnas.0905245106
Kuwahara, A., Ozone, C., Nakano, T., Saito, K., Eiraku, M., & Sasai, Y. (2015a). Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nature Communications, 6(1). https://doi.org/10.1038/ncomms7286
Buchholz, D. E., Hikita, S. T., Rowland, T. J., Friedrich, A. M., Hinman, C. R., Johnson, L. V., & Clegg, D. O. (2009). Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells, 27(10), 2427–2434. https://doi.org/10.1002/stem.189
Liu, S., Xie, B., Song, X., Zheng, D., He, L., Li, G., Gao, G., Peng, F., Yu, M., Ge, J., & Zhong, X. (2018). Self-Formation of RPE spheroids facilitates enrichment and expansion of hiPSC-derived RPE generated on retinal organoid induction platform. Investigative Opthalmology & Visual Science, 59(13), 5659. https://doi.org/10.1167/iovs.17-23613
Isla-Magrané, H., Veiga, A., García-Arumí, J., & Duarri, A. (2021). Multiocular organoids from human induced pluripotent stem cells displayed retinal, corneal, and retinal pigment epithelium lineages. Stem Cell Research & Therapy, 12(1). https://doi.org/10.1186/s13287-021-02651-9
Livesey, F. J., & Cepko, C. L. (2001). Vertebrate neural cell-fate determination: Lessons from the retina. Nature Reviews Neuroscience, 2(2), 109–118. https://doi.org/10.1038/35053522
Young, R. W. (1985). Cell differentiation in the retina of the mouse. The Anatomical Record, 212(2), 199–205. https://doi.org/10.1002/ar.1092120215
Cepko, C. L., Austin, C. P., Yang, X., Alexiades, M., & Ezzeddine, D. (1996). Cell fate determination in the vertebrate retina. Proceedings of the National Academy of Sciences, 93(2), 589–595. https://doi.org/10.1073/pnas.93.2.589
Mao, X., An, Q., Xi, H., Yang, X.-J., Zhang, X., Yuan, S., Wang, J., Hu, Y., Liu, Q., & Fan, G. (2019). Single-cell RNA sequencing of hESC-derived 3D retinal organoids reveals novel genes regulating RPC commitment in early human retinogenesis. Stem Cell Reports, 13(4), 747–760. https://doi.org/10.1016/j.stemcr.2019.08.012
Zhang, X., Mandric, I., Nguyen, K. H., Nguyen, T. T. T., Pellegrini, M., Grove, J. C. R., Barnes, S., & Yang, X.-J. (2021). Single cell transcriptomic analyses reveal the impact of bHLH factors on human retinal organoid development. Frontiers in Cell and Developmental Biology, 9. https://doi.org/10.3389/fcell.2021.653305
Das, G., Choi, Y., Sicinski, P., & Levine, E. M. (2009). Cyclin D1 fine-tunes the neurogenic output of embryonic retinal progenitor cells. Neural Development, 4(1). https://doi.org/10.1186/1749-8104-4-15
Fligor, C. M., Langer, K. B., Sridhar, A., Ren, Y., Shields, P. K., Edler, M. C., Ohlemacher, S. K., Sluch, V. M., Zack, D. J., Zhang, C., Suter, D. M., & Meyer, J. S. (2018). Three-dimensional retinal organoids facilitate the investigation of retinal ganglion cell development, organization and neurite outgrowth from human pluripotent stem cells. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-32871-8
Singh, R. K., Mallela, R. K., Cornuet, P. K., Reifler, A. N., Chervenak, A. P., West, M. D., Wong, K. Y., & Nasonkin, I. O. (2015). Characterization of three-dimensional retinal tissue derived from human embryonic stem cells in adherent monolayer cultures. Stem Cells and Development, 24(23), 2778–2795. https://doi.org/10.1089/scd.2015.0144
Ludwig, A. L., Mayerl, S. J., Gao, Y., Banghart, M., Bacig, C., Zepeda, M. A. F., Zhao, X., & Gamm, D. M. (2023). Re-formation of synaptic connectivity in dissociated human stem cell-derived retinal organoid cultures. Proceedings of the National Academy of Sciences, 120(2). https://doi.org/10.1073/pnas.2213418120
Cora, V., Haderspeck, J., Antkowiak, L., Mattheus, U., Neckel, P., Mack, A., Bolz, S., Ueffing, M., Pashkovskaia, N., Achberger, K., & Liebau, S. (2019). A cleared view on retinal organoids. Cells, 8(5), 391. https://doi.org/10.3390/cells8050391
Knickmeyer, M. D., Mateo, J. L., & Heermann, S. (2021). BMP signaling interferes with optic chiasm formation and retinal ganglion cell pathfinding in zebrafish. International Journal of Molecular Sciences, 22(9), 4560. https://doi.org/10.3390/ijms22094560
Peng, J., Fabre, P. J., Dolique, T., Swikert, S. M., Kermasson, L., Shimogori, T., & Charron, F. (2018). Sonic hedgehog is a remotely produced cue that controls axon guidance trans-axonally at a midline choice point. Neuron, 97(2), 326-340.e4. https://doi.org/10.1016/j.neuron.2017.12.028
Dorgau, B., Felemban, M., Sharpe, A., Bauer, R., Hallam, D., Steel, D. H., Lindsay, S., Mellough, C., & Lako, M. (2018). Laminin γ3 plays an important role in retinal lamination, photoreceptor organisation and ganglion cell differentiation. Cell Death & Disease, 9(6). https://doi.org/10.1038/s41419-018-0648-0
Wagstaff, P. E., Asbroek, A. L. M. A. ten, Brink, J. B. ten, Jansonius, N. M., & Bergen, A. A. B. (2021). An alternative approach to produce versatile retinal organoids with accelerated ganglion cell development. Scientific Reports, 11(1). https://doi.org/10.1038/s41598-020-79651-x
Kaewkhaw, R., Kaya, K. D., Brooks, M., Homma, K., Zou, J., Chaitankar, V., Rao, M., & Swaroop, A. (2015). Transcriptome dynamics of developing photoreceptors in three-dimensional retina cultures recapitulates temporal sequence of human cone and rod differentiation revealing cell surface markers and Gene N. Stem Cells, 33(12), 3504–3518. https://doi.org/10.1002/stem.2122
Kaufman, M. L., Park, K. U., Goodson, N. B., Chew, S., Bersie, S., Jones, K. L., Lamba, D. A., & Brzezinski, J. A. (2019). Transcriptional profiling of murine retinas undergoing semi-synchronous cone photoreceptor differentiation. Developmental Biology, 453(2), 155–167. https://doi.org/10.1016/j.ydbio.2019.05.016
Goodson, N. B., Kaufman, M. A., Park, K. U., & Brzezinski, J. A. (2020). Simultaneous deletion of Prdm1 and Vsx2 enhancers in the retina alters photoreceptor and bipolar cell fate specification, yet differs from deleting both genes. Development. https://doi.org/10.1242/dev.190272
Mears, A. J., Kondo, M., Swain, P. K., Takada, Y., Bush, R. A., Saunders, T. L., Sieving, P. A., & Swaroop, A. (2001). Nrl is required for rod photoreceptor development. Nature Genetics, 29(4), 447–452. https://doi.org/10.1038/ng774
Kim, J.-W., Yang, H.-J., Oel, A. P., Brooks, M. J., Jia, L., Plachetzki, D. C., Li, W., Allison, W. T., & Swaroop, A. (2016). Recruitment of rod photoreceptors from short-wavelength-sensitive cones during the evolution of nocturnal vision in mammals. Developmental Cell, 37(6), 520–532. https://doi.org/10.1016/j.devcel.2016.05.023
Kallman, A., Capowski, E. E., Wang, J., Kaushik, A. M., Jansen, A. D., Edwards, K. L., Chen, L., Berlinicke, C. A., Phillips, M. J., Pierce, E. A., Qian, J., Wang, T.-H., Gamm, D. M., & Zack, D. J. (2020a). Investigating cone photoreceptor development using patient-derived NRL null retinal organoids. Communications Biology, 3(1). https://doi.org/10.1038/s42003-020-0808-5
Xiao, M., & Hendrickson, A. (2000). Spatial and temporal expression of short long medium or both opsins in human. Journal of Comparative Neurology, 5, 545–559. ©2000 WILEY-LISS, INC
Eldred, K. C., Hadyniak, S. E., Hussey, K. A., Brenerman, B., Zhang, P.-W., Chamling, X., Sluch, V. M., Welsbie, D. S., Hattar, S., Taylor, J., Wahlin, K., Zack, D. J., & Johnston, R. J. (2018). Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science, 362(6411). https://doi.org/10.1126/science.aau6348
Rizzolo, L. J., Nasonkin, I. O., & Adelman, R. A. (2022). Retinal Cell Transplantation, Biomaterials, and In Vitro Models for Developing Next-generation Therapies of Age-related Macular Degeneration. Stem Cells Translational Medicine, 11(3), 269–281. https://doi.org/10.1093/stcltm/szac001
Li, J., Chen, Y., Ouyang, S., Ma, J., Sun, H., Luo, L., Chen, S., & Liu, Y. (2021). Generation and staging of human retinal organoids based on self-formed ectodermal autonomous multi-zone system. Frontiers in Cell and Developmental Biology, 9. https://doi.org/10.3389/fcell.2021.732382
Akhtar, T., et al. (2019). Accelerated photoreceptor differentiation of hiPSC-derived retinal organoids by contact co-culture with retinal pigment epithelium. Stem Cell Research, 39, 101491. https://doi.org/10.1016/j.scr.2019.101491
Achberger, K., Probst, C., Haderspeck, J., Bolz, S., Rogal, J., Chuchuy, J., Nikolova, M., Cora, V., Antkowiak, L., Haq, W., Shen, N., Schenke-Layland, K., Ueffing, M., Liebau, S., & Loskill, P. (2019b). Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. ELife, 8. https://doi.org/10.7554/elife.46188
Zhang, Z., Xu, Z., Yuan, F., Jin, K., & Xiang, M. (2021). Retinal organoid technology: Where are we now? International Journal of Molecular Sciences, 22(19), 10244. https://doi.org/10.3390/ijms221910244
Agathon, A., Thisse, C., & Thisse, B. (2003). The molecular nature of the zebrafish tail organizer. Nature, 424(6947), 448–452. https://doi.org/10.1038/nature01822
McNerney, C., & Johnston, R. J. (2021). Thyroid hormone signaling specifies cone photoreceptor subtypes during eye development: Insights from model organisms and human stem cell-derived retinal organoids. In Elsevier (pp. 51–90). https://doi.org/10.1016/bs.vh.2021.03.001
Browne, A. W., Arnesano, C., Harutyunyan, N., Khuu, T., Martinez, J. C., Pollack, H. A., Koos, D. S., Lee, T. C., Fraser, S. E., Moats, R. A., Aparicio, J. G., & Cobrinik, D. (2017). Structural and functional characterization of human stem-cell-derived retinal organoids by live imaging. Retinal Cell Biology. https://doi.org/10.1167/iovs.16-20796
Döpper, H., Menges, J., Bozet, M., Brenzel, A., Lohmann, D., Steenpass, L., & Kanber, D. (2020). Differentiation Protocol for 3D Retinal Organoids, Immunostaining and Signal Quantitation. Current Protocols in Stem Cell Biology, 55(1). https://doi.org/10.1002/cpsc.120
Du, Y., Xiao, Q., & Yip, H. K. (2010). z7g00710003764. Retinal Cell Biology. https://doi.org/10.1167/iovs.09-4906
VanderWall, K. B., Huang, K.-C., Pan, Y., Lavekar, S. S., Fligor, C. M., Allsop, A. R., Lentsch, K. A., Dang, P., Zhang, C., Tseng, H. C., Cummins, T. R., & Meyer, J. S. (2020). Retinal ganglion cells with a glaucoma OPTN(E50K) mutation exhibit neurodegenerative phenotypes when derived from three-dimensional retinal organoids. Stem Cell Reports, 15(1), 52–66. https://doi.org/10.1016/j.stemcr.2020.05.009
Chavarría, T., Valenciano, A. I., Mayordomo, R., Egea, J., Comella, J. X., Hallböök, F., de Pablo, F., & de la Rosa, E. J. (2007). Differential, age-dependent MEK-ERK and PI3K-Akt activation by insulin acting as a survival factor during embryonic retinal development. Developmental Neurobiology, 67(13), 1777–1788. https://doi.org/10.1002/dneu.20554
Braunger, B. M., Pielmeier, S., Demmer, C., Landstorfer, V., Kawall, D., Abramov, N., Leibinger, M., Kleiter, I., Fischer, D., Jagle, H., & Tamm, E. R. (2013). TGF- signaling protects retinal neurons from programmed cell death during the development of the Mammalian eye. Journal of Neuroscience, 33(35), 14246–14258. https://doi.org/10.1523/jneurosci.0991-13.2013
Chavali, V. R. M., Haider, N., Rathi, S., Vrathasha, V., Alapati, T., He, J., Gill, K., Nikonov, R., Duong, T. T., McDougald, D. S., Nikonov, S., O’Brien, J., & Mills, J. A. (2020). Dual SMAD inhibition and Wnt inhibition enable efficient and reproducible differentiations of induced pluripotent stem cells into retinal ganglion cells. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-68811-8
Chambers, S. M., Fasano, C. A., Papapetrou, E. P., Tomishima, M., Sadelain, M., & Studer, L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotechnology, 27(3), 275–280. https://doi.org/10.1038/nbt.1529
Belloni, E., Muenke, M., Roessler, E., Traverse, G., Siegel-Bartelt, J., Frumkin, A., Mitchell, H. F., Donis-Keller, H., Helms, C., Hing, A. V., Heng, H. H. Q., Koop, B., Martindale, D., Rommens, J. M., Tsui, L.-C., & Scherer, S. W. (1996). Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nature Genetics, 14(3), 353–356. https://doi.org/10.1038/ng1196-353
Stenkamp, D. L. (2015). Development of the Vertebrate Eye and Retina. In Elsevier (pp. 397–414). https://doi.org/10.1016/bs.pmbts.2015.06.006
Neumann, C. J., & Nuesslein-Volhard, C. (2000). Patterning of the zebrafish retina by a wave of sonic hedgehog activity. Science, 289(5487), 2137–2139. https://doi.org/10.1126/science.289.5487.2137
Wiley, L. A., Burnight, E. R., DeLuca, A. P., Anfinson, K. R., Cranston, C. M., Kaalberg, E. E., Penticoff, J. A., Affatigato, L. M., Mullins, R. F., Stone, E. M., & Tucker, B. A. (2016). cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Scientific Reports, 6(1). https://doi.org/10.1038/srep30742
Toonen, J. A., Ronchetti, A., & Sidjanin, D. J. (2016). A disintegrin and metalloproteinase10 (ADAM10) regulates NOTCH signaling during early retinal development. PLOS ONE, 11(5), e0156184. https://doi.org/10.1371/journal.pone.0156184
Jadhav, A. P., Cho, S.-H., & Cepko, C. L. (2006). Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proceedings of the National Academy of Sciences, 103(50), 18998–19003. https://doi.org/10.1073/pnas.0608155103
Mochizuki, Y., Iida, A., Lyons, E., Kageyama, R., Nakauchi, H., Murakami, A., & Watanabe, S. (2013). Use of cell type-specific transcriptome to identify genes specifically involved in Müller glia differentiation during retinal development. Developmental Neurobiology, 74(4), 426–437. https://doi.org/10.1002/dneu.22131
Mizeracka, K., Trimarchi, J. M., Stadler, M. B., & Cepko, C. L. (2013). Analysis of gene expression in wild-type and Notch1 mutant retinal cells by single cell profiling. Developmental Dynamics, 242(10), 1147–1159. https://doi.org/10.1002/dvdy.24006
Shrestha, R., Wen, Y.-T., Ding, D.-C., & Tsai, R.-K. (2019). Aberrant hiPSCs-derived from human keratinocytes differentiates into 3D retinal organoids that acquire mature photoreceptors. Cells, 8(1), 36. https://doi.org/10.3390/cells8010036
Wahlin, K. J., Maruotti, J. A., Sripathi, S. R., Ball, J., Angueyra, J. M., Kim, C., Grebe, R., Li, W., Jones, B. W., & Zack, D. J. (2017). Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-00774-9
Zerti, D., Dorgau, B., Felemban, M., Ghareeb, A. E., Yu, M., Ding, Y., Krasnogor, N., & Lako, M. (2019). Developing a simple method to enhance the generation of cone and rod photoreceptors in pluripotent stem cell-derived retinal organoids. Stem Cells, 38(1), 45–51. https://doi.org/10.1002/stem.3082
Achberger, K. (2019). Abstract.https://doi.org/10.7554/elife.46188.001
Yeste, J., García-Ramírez, M., Illa, X., Guimerà, A., Hernández, C., Simó, R., & Villa, R. (2018). A compartmentalized microfluidic chip with crisscross microgrooves and electrophysiological electrodes for modeling the blood–retinal barrier. Lab on a Chip, 18(1), 95–105. https://doi.org/10.1039/c7lc00795g
Xue, Y., Seiler, M. J., Tang, W. C., Wang, J. Y., Delgado, J., McLelland, B. T., Nistor, G., Keirstead, H. S., & Browne, A. W. (2021). Retinal organoids on-a-chip: A micro-millifluidic bioreactor for long-term organoid maintenance. Lab on a Chip, 21(17), 3361–3377. https://doi.org/10.1039/d1lc00011j
Lipecz, A., Miller, L., Kovacs, I., Czakó, C., Csipo, T., Baffi, J., Csiszar, A., Tarantini, S., Ungvari, Z., Yabluchanskiy, A., & Conley, S. (2019). Microvascular contributions to age-related macular degeneration (AMD): From mechanisms of choriocapillaris aging to novel interventions. GeroScience, 41(6), 813–845. https://doi.org/10.1007/s11357-019-00138-3
Chung, M., Lee, S., Lee, B. J., Son, K., Jeon, N. L., & Kim, J. H. (2017). Wet-AMD on a chip: modeling outer blood-retinal barrier in vitro. Advanced Healthcare Materials, 7(2), 1700028. https://doi.org/10.1002/adhm.201700028
Chen, L.-J., Ito, S., Kai, H., Nagamine, K., Nagai, N., Nishizawa, M., Abe, T., & Kaji, H. (2017). Microfluidic co-cultures of retinal pigment epithelial cells and vascular endothelial cells to investigate choroidal angiogenesis. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-03788-5
Pașca, S. P., Arlotta, P., Bateup, H. S., Camp, J. G., Cappello, S., Gage, F. H., Knoblich, J. A., Kriegstein, A. R., Lancaster, M. A., Ming, G.-L., Muotri, A. R., Park, I.-H., Reiner, O., Song, H., Studer, L., Temple, S., Testa, G., Treutlein, B., & Vaccarino, F. M. (2022). A nomenclature consensus for nervous system organoids and assembloids. Nature, 609(7929), 907–910. https://doi.org/10.1038/s41586-022-05219-6
Fernando, M., Lee, S., Wark, J. R., Xiao, D., Lim, B. Y., O’Hara-Wright, M., Kim, H. J., Smith, G. C., Wong, T., Teber, E. T., Ali, R. R., Yang, P., Graham, M. E., & Gonzalez-Cordero, A. (2022). Differentiation of brain and retinal organoids from confluent cultures of pluripotent stem cells connected by nerve-like axonal projections of optic origin. Stem Cell Reports, 17(6), 1476–1492. https://doi.org/10.1016/j.stemcr.2022.04.003
Mansour, A. A., Gonçalves, J. T., Bloyd, C. W., Li, H., Fernandes, S., Quang, D., Johnston, S., Parylak, S. L., Jin, X., & Gage, F. H. (2018). An in vivo model of functional and vascularized human brain organoids. Nature Biotechnology, 36(5), 432–441. https://doi.org/10.1038/nbt.4127
Brooks, M. J., Chen, H. Y., Kelley, R. A., Mondal, A. K., Nagashima, K., Val, N. D., Li, T., Chaitankar, V., & Swaroop, A. (2019). Improved retinal organoid differentiation by modulating signaling pathways revealed by comparative transcriptome analyses with development in vivo. Stem Cell Reports, 13(5), 891–905. https://doi.org/10.1016/j.stemcr.2019.09.009
Sridhar, A., Hoshino, A., Finkbeiner, C. R., Chitsazan, A., Dai, L., Haugan, A. K., Eschenbacher, K. M., Jackson, D. L., Trapnell, C., Bermingham-McDonogh, O., Glass, I., & Reh, T. A. (2020). Single-cell transcriptomic comparison of human fetal retina, hPSC-derived retinal organoids, and long-term retinal cultures. Cell Reports, 30(5), 1644-1659.e4. https://doi.org/10.1016/j.celrep.2020.01.007
Finkbeiner, C., Ortuño-Lizarán, I., Sridhar, A., Hooper, M., Petter, S., & Reh, T. A. (2022). Single-cell ATAC-seq of fetal human retina and stem-cell-derived retinal organoids shows changing chromatin landscapes during cell fate acquisition. Cell Reports, 38(4), 110294. https://doi.org/10.1016/j.celrep.2021.110294
Xie, H., Zhang, W., Zhang, M., Akhtar, T., Li, Y., Yi, W., Sun, X., Zuo, Z., Wei, M., Fang, X., Yao, Z., Dong, K., Zhong, S., Liu, Q., Shen, Y., Wu, Q., Wang, X., Zhao, H., Bao, J., Xue, T. (2020). Chromatin accessibility analysis reveals regulatory dynamics of developing human retina and hiPSC-derived retinal organoids. Science Advances, 6(6). https://doi.org/10.1126/sciadv.aay5247
Thomas, E. D., Timms, A. E., Giles, S., Harkins-Perry, S., Lyu, P., Hoang, T., Qian, J., Jackson, V. E., Bahlo, M., Blackshaw, S., Friedlander, M., Eade, K., & Cherry, T. J. (2022). Cell-specific cis-regulatory elements and mechanisms of non-coding genetic disease in human retina and retinal organoids. Developmental Cell, 57(6), 820-836.e6. https://doi.org/10.1016/j.devcel.2022.02.018
Chen, A., Liao, S., Cheng, M., Ma, K., Wu, L., Lai, Y., Qiu, X., Yang, J., Xu, J., Hao, S., Wang, X., Lu, H., Chen, X., Liu, X., Huang, X., Li, Z., Hong, Y., Jiang, Y., Peng, J., Wang, J. (2022). Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell, 185(10), 1777–1792.e21. https://doi.org/10.1016/j.cell.2022.04.003
Dias, M. F., Joo, K., Kemp, J. A., Fialho, S. L., Cunha, A., da S., Woo, S. J., & Kwon, Y. J. (2018). Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Progress in Retinal and Eye Research, 63, 107–131https://doi.org/10.1016/j.preteyeres.2017.10.004
Deng, W.-L., Gao, M.-L., Lei, X.-L., Lv, J.-N., Zhao, H., He, K.-W., Xia, X.-X., Li, L.-Y., Chen, Y.-C., Li, Y.-P., Pan, D., Xue, T., & Jin, Z.-B. (2018). Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Reports, 10(4), 1267–1281. https://doi.org/10.1016/j.stemcr.2018.02.003
Karam, F. C., Loi, T. H., Ma, A., Nash, B. M., Grigg, J. R., Parekh, D., Riley, L. G., Farnsworth, E., Bennetts, B., Gonzalez-Cordero, A., & Jamieson, R. V. (2022). Human iPSC-derived retinal organoids and retinal pigment epithelium for novel intronic RPGR variant assessment for therapy suitability. Journal of Personalized Medicine, 12(3), 502. https://doi.org/10.3390/jpm12030502
Buskin, A., Zhu, L., Chichagova, V., Basu, B., Mozaffari-Jovin, S., Dolan, D., Droop, A., Collin, J., Bronstein, R., Mehrotra, S., Farkas, M., Hilgen, G., White, K., Pan, K.-T., Treumann, A., Hallam, D., Bialas, K., Chung, G., Mellough, C., Lako, M. (2018). Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nature Communications, 9(1). https://doi.org/10.1038/s41467-018-06448-y
Georgiou, M., Yang, C., Atkinson, R., Pan, K., Buskin, A., Molina, M. M., Collin, J., Al‐Aama, J., Goertler, F., Ludwig, S. E. J., Davey, T., Lührmann, R., Nagaraja‐Grellscheid, S., Johnson, C. A., Ali, R., Armstrong, L., Korolchuk, V., Urlaub, H., Mozaffari‐Jovin, S., & Lako, M. (2022). Activation of autophagy reverses progressive and deleterious protein aggregation in PRPF31 patient‐induced pluripotent stem cell‐derived retinal pigment epithelium cells. Clinical and Translational Medicine, 12(3). https://doi.org/10.1002/ctm2.759
Guo, Y., Wang, P., Ma, J. H., Cui, Z., Yu, Q., Liu, S., Xue, Y., Zhu, D., Cao, J., Li, Z., Tang, S., & Chen, J. (2019). Modeling retinitis pigmentosa: Retinal organoids generated from the iPSCs of a patient with the USH2A mutation show early developmental abnormalities. Frontiers in Cellular Neuroscience, 13. https://doi.org/10.3389/fncel.2019.00361
Martínez-Sánchez, M., Hernandez-Monge, J., Rangel, M., & Olivares-Illana, V. (2021). Retinoblastoma: From discovery to clinical management. The FEBS Journal, 289(15), 4371–4382. https://doi.org/10.1111/febs.16035
Liu, H., Zhang, Y., Zhang, Y.-Y., Li, Y.-P., Hua, Z.-Q., Zhang, C.-J., Wu, K.-C., Yu, F., Zhang, Y., Su, J., & Jin, Z.-B. (2020). Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proceedings of the National Academy of Sciences, 117(52), 33628–33638. https://doi.org/10.1073/pnas.2011780117
Kanber, D., Woestefeld, J., Döpper, H., Bozet, M., Brenzel, A., Altmüller, J., Kilpert, F., Lohmann, D., Pommerenke, C., & Steenpass, L. (2022). RB1-negative retinal organoids display proliferation of cone photoreceptors and loss of retinal differentiation. Cancers, 14(9), 2166. https://doi.org/10.3390/cancers14092166
Blixt, M. K. E., Hellsand, M., Konjusha, D., Zhang, H., Stenfelt, S., Åkesson, M., Rafati, N., Tararuk, T., Stålhammar, G., All-Eriksson, C., Ring, H., & Hallböök, F. (2022). MYCN induces cell-specific tumorigenic growth in RB1-proficient human retinal organoid and chicken retina models of retinoblastoma. Oncogenesis, 11(1). https://doi.org/10.1038/s41389-022-00409-3
Lukovic, D., Castro, A. A., Kaya, K. D., Munezero, D., Gieser, L., Davó-Martínez, C., Corton, M., Cuenca, N., Swaroop, A., Ramamurthy, V., Ayuso, C., & Erceg, S. (2020). Retinal Organoids derived from hiPSCs of an AIPL1-LCA Patient Maintain Cytoarchitecture despite Reduced levels of Mutant AIPL1. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-62047-2
Li, G., Gao, G., Wang, P., Song, X., Xu, P., Xie, B., Zhou, T., Pan, G., Peng, F., Zhang, Q., Ge, J., & Zhong, X. (2019). Generation and Characterization of Induced Pluripotent Stem Cells and Retinal Organoids From a Leber’s Congenital Amaurosis Patient With Novel RPE65 Mutations. Frontiers in Molecular Neuroscience, 12. https://doi.org/10.3389/fnmol.2019.00212
Kruczek, K., Qu, Z., Gentry, J., Fadl, B. R., Gieser, L., Hiriyanna, S., Batz, Z., Samant, M., Samanta, A., Chu, C. J., Campello, L., Brooks, B. P., Wu, Z., & Swaroop, A. (2021). Gene therapy of dominant CRX-Leber congenital amaurosis using patient stem cell-derived retinal organoids. Stem Cell Reports, 16(2), 252–263. https://doi.org/10.1016/j.stemcr.2020.12.018
Gonzalez-Cordero, A., Kruczek, K., Naeem, A., Fernando, M., Kloc, M., Ribeiro, J., Goh, D., Duran, Y., Blackford, S. J. I., Abelleira-Hervas, L., Sampson, R. D., Shum, I. O., Branch, M. J., Gardner, P. J., Sowden, J. C., Bainbridge, J. W. B., Smith, A. J., West, E. L., Pearson, R. A., & Ali, R. R. (2017). Recapitulation of human retinal development from human pluripotent stem cells generates transplantable populations of cone photoreceptors. Stem Cell Reports, 9(3), 820–837. https://doi.org/10.1016/j.stemcr.2017.07.022
Pearson, R. A., Gonzalez-Cordero, A., West, E. L., Ribeiro, J. R., Aghaizu, N., Goh, D., Sampson, R. D., Georgiadis, A., Waldron, P. V., Duran, Y., Naeem, A., Kloc, M., Cristante, E., Kruczek, K., Warre-Cornish, K., Sowden, J. C., Smith, A. J., & Ali, R. R. (2016). Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nature Communications, 7(1). https://doi.org/10.1038/ncomms13029
Pearson, R. A., Barber, A. C., Rizzi, M., Hippert, C., Xue, T., West, E. L., Duran, Y., Smith, A. J., Chuang, J. Z., Azam, S. A., Luhmann, U. F. O., Benucci, A., Sung, C. H., Bainbridge, J. W., Carandini, M., Yau, K.-W., Sowden, J. C., & Ali, R. R. (2012). Restoration of vision after transplantation of photoreceptors. Nature, 485(7396), 99–103. https://doi.org/10.1038/nature10997
Gonzalez-Cordero, A., West, E. L., Pearson, R. A., Duran, Y., Carvalho, L. S., Chu, C. J., Naeem, A., Blackford, S. J. I., Georgiadis, A., Lakowski, J., Hubank, M., Smith, A. J., Bainbridge, J. W. B., Sowden, J. C., & Ali, R. R. (2013). Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nature Biotechnology, 31(8), 741–747. https://doi.org/10.1038/nbt.2643
Waldron, P. V., Marco, F. D., Kruczek, K., Ribeiro, J., Graca, A. B., Hippert, C., Aghaizu, N. D., Kalargyrou, A. A., Barber, A. C., Grimaldi, G., Duran, Y., Blackford, S. J. I., Kloc, M., Goh, D., Aldunate, E. Z., Sampson, R. D., Bainbridge, J. W. B., Smith, A. J., Gonzalez-Cordero, A., Pearson, R. A. (2018). Transplanted donor- or stem cell-derived cone photoreceptors can both integrate and undergo material transfer in an environment-dependent manner. Stem Cell Reports, 10(2), 406–421. https://doi.org/10.1016/j.stemcr.2017.12.008
Lin, B., McLelland, B. T., Aramant, R. B., Thomas, B. B., Nistor, G., Keirstead, H. S., & Seiler, M. J. (2020). Retina organoid transplants develop photoreceptors and improve visual function in RCS rats with RPE dysfunction. Investigative Opthalmology & Visual Science, 61(11), 34. https://doi.org/10.1167/iovs.61.11.34
Zhu, D., Xie, M., Gademann, F., Cao, J., Wang, P., Guo, Y., Zhang, L., Su, T., Zhang, J., & Chen, J. (2020). Protective effects of human iPS-derived retinal pigmented epithelial cells on retinal degenerative disease. Stem Cell Research & Therapy, 11(1). https://doi.org/10.1186/s13287-020-01608-8
Mandai, M., Fujii, M., Hashiguchi, T., Sunagawa, G. A., Ito, S., Sun, J., Kaneko, J., Sho, J., Yamada, C., & Takahashi, M. (2017). iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Reports, 8(1), 69–83. https://doi.org/10.1016/j.stemcr.2016.12.008
Oswald, J., Kegeles, E., Minelli, T., Volchkov, P., & Baranov, P. (2021). Transplantation of miPSC/mESC-derived retinal ganglion cells into healthy and glaucomatous retinas. Molecular Therapy - Methods & Clinical Development, 21, 180–198. https://doi.org/10.1016/j.omtm.2021.03.004
Wang, S.-T., Chen, L., Zhang, P., Wang, X.-B., Sun, Y., Ma, L.-X., Liu, Q., & Zhou, G.-M. (2019). Transplantation of retinal progenitor cells from optic cup-like structures differentiated from human embryonic stem cells in vitro and in vivo generation of retinal ganglion-like cells. Stem Cells and Development, 28(4), 258–267. https://doi.org/10.1089/scd.2018.0076
Zou, T., Gao, L., Zeng, Y., Li, Q., Li, Y., Chen, S., Hu, X., Chen, X., Fu, C., Xu, H., & Yin, Z. Q. (2019a). Organoid-derived C-Kit+/SSEA4− human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-08961-0
Singh, R. K., Occelli, L. M., Binette, F., Petersen-Jones, S. M., & Nasonkin, I. O. (2019). Transplantation of human embryonic stem cell-derived retinal tissue in the subretinal space of the cat eye. Stem Cells and Development, 28(17), 1151–1166. https://doi.org/10.1089/scd.2019.0090
Uhl, E. W., & Warner, N. J. (2015). Mouse models as predictors of human responses: Evolutionary medicine. Current Pathobiology Reports, 3(3), 219–223. https://doi.org/10.1007/s40139-015-0086-y
Renner, H., Grabos, M., Becker, K. J., Kagermeier, T. E., Wu, J., Otto, M., Peischard, S., Zeuschner, D., TsyTsyura, Y., Disse, P., Klingauf, J., Leidel, S. A., Seebohm, G., Schöler, H. R., & Bruder, J. M. (2020). A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids. ELife, 9. https://doi.org/10.7554/elife.52904
Parihar, A., Pandita, V., & Khan, R. (2022). 3D printed human organoids: High throughput system for drug screening and testing in current COVID-19 pandemic. Biotechnology and Bioengineering, 119(10), 2669–2688. https://doi.org/10.1002/bit.28166
Schuster, B., Junkin, M., Kashaf, S. S., Romero-Calvo, I., Kirby, K., Matthews, J., Weber, C. R., Rzhetsky, A., White, K. P., & Tay, S. (2020). Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nature Communications, 11(1). https://doi.org/10.1038/s41467-020-19058-4
Hou, S., Tiriac, H., Sridharan, B. P., Scampavia, L., Madoux, F., Seldin, J., Souza, G. R., Watson, D., Tuveson, D., & Spicer, T. P. (2018). Advanced development of primary pancreatic organoid tumor models for high-throughput phenotypic drug screening. SLAS Discovery, 23(6), 574–584. https://doi.org/10.1177/2472555218766842
Li, X., Fu, G., Zhang, L., Guan, R., Tang, P., Zhang, J., Rao, X., Chen, S., Xu, X., Zhou, Y., Deng, Y., Lv, T., He, X., Mo, S., Mu, P., Gao, J., & Hua, G. (2022). Assay establishment and validation of a high-throughput organoid-based drug screening platform. Stem Cell Research & Therapy, 13(1). https://doi.org/10.1186/s13287-022-02902-3
Saito, Y., Muramatsu, T., Kanai, Y., Ojima, H., Sukeda, A., Hiraoka, N., Arai, E., Sugiyama, Y., Matsuzaki, J., Uchida, R., Yoshikawa, N., Furukawa, R., & Saito, H. (2019). Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Reports, 27(4), 1265-1276.e4. https://doi.org/10.1016/j.celrep.2019.03.088
Funding
This work was supported by the National Natural Science Foundation of China grants 31930068; the National Key Research and Development Program of China grants 2021YFA1101203.
Author information
Authors and Affiliations
Contributions
Conceptualization: Professor Haiwei Xu; Funding acquisition: Professor Haiwei Xu; Writing—original draft preparation: Jiahui Kang; Writing—review and editing:Yu Gong, Jing Gong, Cao Yang, Xi Lin, Lijuan Yan. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, J., Gong, J., Yang, C. et al. Application of Human Stem Cell Derived Retinal Organoids in the Exploration of the Mechanisms of Early Retinal Development. Stem Cell Rev and Rep 19, 1755–1772 (2023). https://doi.org/10.1007/s12015-023-10553-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10553-x