Abstract
The evolution of pluripotent stem cell-derived retinal organoids (ROs) has brought remarkable opportunities for developmental studies while also presenting new therapeutic avenues for retinal diseases. With a clear understanding of how well these models mimic native retinas, such preclinical models may be crucial tools that are widely used for the more efficient translation of studies into novel treatment strategies for retinal diseases. Genetic modifications or patient-derived ROs can allow these models to simulate the physical microenvironments of the actual disease process. However, we are currently at the beginning of the three-dimensional (3D) RO era, and a general quantitative technology for analyzing ROs derived from numerous differentiation protocols is still missing. Continued efforts to improve the efficiency and stability of differentiation, as well as understanding the disparity between the artificial retina and the native retina and advancing the current treatment strategies, will be essential in ensuring that these scientific advances can benefit patients with retinal disease. Herein, we briefly discuss RO differentiation protocols, the current applications of RO as a disease model and the treatments for retinal diseases by using RO modeling, to have a clear view of the role of current ROs in retinal development and diseases.
Similar content being viewed by others
Background
Over the past several decades, our knowledge of retinal diseases has significantly increased (Khan et al. 2016; Veleri et al. 2015). Despite substantial progress in the treatment of inherited retinal disease, it is still a major health problem throughout the world. One of the hurdles for translating scientific knowledge from laboratory settings to clinical settings is the lack of preclinical models. Although mouse models have significantly improved our insights into the basic concepts of retinal diseases, these models may not faithfully recapitulate pathogenic processes in patients, due to species differences (Singh et al. 2018). Currently, human pluripotent stem cell (hPSC)-derived retinal organoids (ROs) have become frequently utilized as 3D models of human retinal development and diseases, due to their easy accessibility, high stability, and proximity to native retinas (Zhang and Jin 2021).
As RO-derived retinal cells are produced in a dish, it is unlimited from a theoretical aspect. From a treatment perspective, ROs are a perfect source of retinal cells for transplantation in cell therapy and an attractive model for validating gene therapies. In recent studies, RO has also been used as a model to identify the mechanisms of inherited retinal degeneration diseases (Buskin et al. 2018; de Bruijn et al. 2020; Deng et al. 2018; Diakatou et al. 2021; Gao et al. 2020; Guo et al. 2019; Kallman et al. 2020; Kruczek et al. 2021; Lane et al. 2020; Li et al. 2019; Lukovic et al. 2020; Quinn et al. 2019; Sharma et al. 2017; Shimada et al. 2017; Zhang et al. 2020b; Zhang et al. 2021) and to test the efficiency of Adeno-associated virus (AAV) infection for retinal cells (Gonzalez-Cordero et al. 2018; Tornabene et al. 2019; Volkner et al. 2021). Additionally, RO has been used to improve the outcomes of gene therapy, to explore the membrane trafficking efficacy of some microbial opsins for cones (Garita-Hernandez et al. 2021; Garita-Hernandez et al. 2018) and to discover photoreceptor cell surface markers (Gagliardi et al. 2018; Santos-Ferreira et al. 2016) that can improve the outcomes of cell therapy. In this review, we briefly summarize the studies that have perfected the RO system, and research that using the RO as a model to enrich the knowledge about retinal development and disease; additionally, we discuss the importance of future therapeutic strategies.
Main text
Brief history of mouse retinal development and maturation in vivo
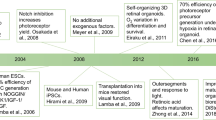
Rodent models have been the most widely used models in retina research for almost a century. These models have significantly contributed to the development, structure and function of retinal cells and their associated diseases (Keeler et al. 1928). From the year of 1980, rodent models have been used as a retinal disease model (Aguirre 1980; Albert 1980). Subsequently, the transgenic mouse disease model was developed in 1990 on retinoblastoma, which greatly encouraged mechanistic research on inherited retinal diseases for many years (Jin et al. 2014; O'Brien et al. 1990; Olsson et al. 1992; Ramamurthy et al. 2004) (Fig. 1). Due to the mouse models, we developed a complete understanding of the seven major retinal cells, including six neuronal cells (rod, cone, horizontal, amacrine, bipolar and ganglion cells) and one glial cell (Müller cell), as well as the two synaptic layers (the outer plexiform layer, OPL and the inner plexiform layer IPL) (Zhang et al. 2011). Nevertheless, the lack of human-specific features in mouse models still hampers further research on clinical applications. From 2012 onwards, 3D-cultured retinal organoids, as an emerging disease model, have become a powerful supplement to mouse models and are becoming favorable for use by scientists (Marshall and Mason 2019) (Fig. 1).
The comparison of the mouse retinal models and the human retinal organoids as models. Mouse models were used as a retinal disease model from year 1980 and transgenic mouse used to model inherited retinal disease from year 1990. In the past decades, a great increase was observed in the studies on human retinal organoids, in contrast, the studies used the mouse for retinal development models are downward
Production of retinal organoids from human pluripotent stem cells
Generation of retinal cell
Photoreceptors were initially generated from hPSCs in a 2D and small-molecule induction differentiation method by the Masayo Takahashi group (Hirami et al. 2009; Osakada et al. 2009). Recently, photoreceptors have been reprogrammed from fibroblasts by using small molecules (Mahato et al. 2020). All of the molecules express specific markers and display the light response to photoreceptors, thus demonstrating an advanced degree of functionality (Hirami et al. 2009; Mahato et al. 2020; Osakada et al. 2009). The method of retinal ganglion cell (RGC) differentiation occurs at a later time than photoreceptor differentiation, which is likely due to the lack of specific RGC markers (Ohlemacher et al. 2019). It involves a stepwise differentiation method, which is characterized by a suspension culture stage with subsequent 2D adhesion culture (Ohlemacher et al. 2016; Tanaka et al. 2015). However, 2D RGCs are still combined with photoreceptors (Chen et al. 2019; Tanaka et al. 2015). Protocols for generating other retinal cells solely from human PSCs have rarely been reported. Moreover, the most common strategy for generating human retinal cells is via RO differentiation, which efficiently recapitulates the lamination structure and function of the retina (Kruczek and Swaroop 2020; Volkner et al. 2016).
Protocols of retinal organoid differentiation
As a model, ROs have been a part of the field for retinal development and retinal disease models for approximately 10 years. In the past decade, numerous protocols for RO differentiation have been established, based on two main approaches: 3D (Nakano et al. 2012) and a combination of 2D and 3D (Lowe et al. 2016; Zhong et al. 2014) methods. The major difference is the embryoid body (EB) stage. The 3D culture system begins with the EB stage and is followed by the optic vesicle (OV) and optic cup stages, whereas the 2D/3D culture system bypasses the EB stage and generates the OV stage from adherent retinal cells (Nakano et al. 2012; Zhong et al. 2014). The different protocols also lead to the different results and currently it is still hard to reach a consensus on the standard for the RO differentiation, in order to develop the quantitative technology for analyzing ROs, we still need time to collect more information to eliminate the effects caused by the objective and subjective variables during the RO differentiation.
There are also some modified protocols that are based on the two previously described approaches that can be summarized into three categories: chemical modification, material modification and coculture modification protocols. 1) The chemical modification protocol involves docosahexaenoic acid and fibroblast growth factor 1, which can specifically promote the maturation of photoreceptors in ROs (Brooks et al. 2019). Exogenous IGF-1 has a positive role in the RO lamination structure, and photoreceptor development relies on IGF-binding proteins (Mellough et al. 2015; Zerti et al. 2021). The combination of retinoic acid (RA), levodopa (l-DOPA), triiodothyronine (T3) and the γ-secretase inhibitor DAPT at different differentiation timepoints can lead to the direct generation of rod and cone photoreceptors in vitro (Zerti et al. 2020). Moreover, Dickkopf-related protein 1 (DKK-1), which is a Wnt signaling pathway antagonist, can induce retinal progenitors to self-organize (Luo et al. 2018). COCO, which is a multifunctional antagonist of the Wnt, TGF-β and BMP pathways, has been proven to efficiently improve the differentiation efficiency of photoreceptor precursors and cones (Pan et al. 2020; Zhou et al. 2015). Obviously, Wnt signaling plays an important role in retinal development. In addition, it significantly contributes to ocular angiogenesis, which indicates that research on Wnt signaling regulation may help in overcoming the challenges of RO vascularization (Sarin et al. 2018; Wang et al. 2019). 2) Material modifications involving bioreactors (DiStefano et al. 2018; Ovando-Roche et al. 2018) and retina-on-chip (Achberger et al. 2019; Hofer and Lutolf 2021; Manafi et al. 2021; Mittal et al. 2019), which are facilities that are used to improve the differentiation efficiency, especially retina-on-chip, which uses microfluidics to mimic the vascular energy supply. This application shows great potential in retinal disease modeling and in ophthalmology translational applications (Achberger et al. 2019; Manafi et al. 2021). 3) Coculture modification can also be used as a modified protocol. Although chemical modification and material modification can somehow enhance the RO differentiation efficiency and promote the stratified RO structure and maturation of photoreceptors, some physical cell-cell interactions are still missing in vivo, such as the RPE-photoreceptor interface, RPE-Bruch’s-choriocapillaris interactions and vascular elements, as well as microglia. The coculture system has achieved the RPE-photoreceptor interface (Achberger et al. 2019; Akhtar et al. 2019) and the RPE-Bruch’s-choriocapillaris interaction (Manian et al. 2021). However, these interfaces are only partially connected, and the integration of the RO with RPE and Bruch’s choriocapillaris, as well as microglia, will be a future area of study for regeneration medicine (Ghareeb et al. 2020).
RO in retinal development
How the RO recapitulates the human in vivo retina
RO has the capability to generate the seven main major retinal cells (Collin et al. 2019; Freude et al. 2020; Kuwahara et al. 2015; Nakano et al. 2012; Zhong et al. 2014) and recapitulate retinogenesis (Mao et al. 2019; Volkner et al. 2016). Recently, the transcriptome profiling of RO and the human retina has displayed considerable similarity (Cowan et al. 2020; Sridhar et al. 2020). The cell types, specific cell differentiation markers, retinal disease genes and alternative mRNA splicing of maturing ROs are consistent with those of human retinas (Collin et al. 2019; Cowan et al. 2020; Kim et al. 2019; Mao et al. 2019; Sridhar et al. 2020). Interestingly, the cones from retinal organoids have transcriptomes that are similar to those of the human macula (Kim et al. 2019); however, the RO presents a relatively inferior inner retina lamination than the human retinas (Sridhar et al. 2020). Taken together, the retinal cell type of RO is the same as that of human retinas, but the proportions of the seven retinal cells are still different from those of human retinas. Moreover, there are more results observed on the outer retina, especially on the photoreceptors. Currently, the structure of the out retina is more similar to the native retina than the inner retina and further studies on the inner retina is necessary to perfect the retinal organoid models.
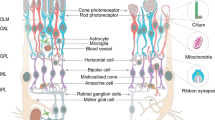
Seven major retinal cells in the human retinal organoids
Photoreceptors (cones and rods) are the retinal cells in ROs that possess the most details. The outer segments connected cilia and inner segments are all well displayed via transmission electron microscopy (Capowski et al. 2019; Mellough et al. 2015) and specific marker expression studies (Nakano et al. 2012; Pan et al. 2020). The typical discs of the outer segments, which are regulated by the photoreceptor cilium actin regulator C2orf71/PCARE and complex activator WASF3 (Corral-Serrano et al. 2020), also become a feature of the mature photoreceptors in ROs. In addition, the cell surface antigen CD73 has been demonstrated to be a marker for transplantable photoreceptors (Gagliardi et al. 2018; Santos-Ferreira et al. 2016). Research on rods and cones is also an interesting process. For example, rods are isolated by using real-time deformability cytometry with unique mechanical and morphological features during development, rather than isolation with the use of labels (Santos-Ferreira et al. 2019). Furthermore, thyroid hormone is identified as being a main regulator of the formation of the S-cone or M/L-cone, low levels of thyroid hormone signaling at the early stages of development can promote the S fate, and high levels of thyroid hormone signaling at late stages can promote the L/M fate (Eldred et al. 2018). The data for horizontal, amacrine and bipolar cells are relatively less than those for photoreceptors in ROs, with results only being demonstrated in cell identification during human RO differentiation (Cowan et al. 2020; Nakano et al. 2012; Zhong et al. 2014). Studies on differentiated RGCs are confluent; they can be directly induced into RGCs from retinal progenitor cells (Chavali et al. 2020; Zhang et al. 2020a) or generated as retinal cells in ROs by using the 3D/2D stepwise differentiation protocol (Chen et al. 2019; Fligor et al. 2018; Freude et al. 2020; Kobayashi et al. 2018; Rabesandratana et al. 2020), or purified from ROs (Fligor et al. 2021). RGCs in those methods all display neurite outgrowth (Chavali et al. 2020; Fligor et al. 2018; Fligor et al. 2021). Notably, Müller cells (more specifically, the factors released by Müller cells) play an important role in PSC-derived RGC survival and neuritogenesis (Pereiro et al. 2020). Early RGCs and RGCs cells can be isolated via the cell surface markers CD90 and CD171, Müller cells can be isolated via the cell surface markers CD44 and CD117, which can broadly benefit cell replacement therapies related to RGCs and Müller cells (Aparicio et al. 2017; Chavali et al. 2020; Freude et al. 2020; Shinoe et al. 2010; Too et al. 2017) (Table 1).
Two synaptic layers in the human retinal organoids
The two synaptic layers contain photoreceptors that synapse onto bipolar cells, and the bipolar cells synapse onto ganglion cells. It is well known that there are OFF and ON bipolar cells, with these cells synapsing on OFF-center and ON-center ganglion cells, respectively, in the retina. Remarkably, ROs at day 150 not only form synapses, but also exhibit a phototransduction cascade and synaptic circuitry (Cowan et al. 2020; Dorgau et al. 2019; Hallam et al. 2018). Repeatable light responses can also be detected in the outer nuclear layer, 12% inner nuclear layer and RGC layers (Cowan et al. 2020; Kim et al. 2019). Additionally, these photoreceptors hyperpolarize in response to light, which indicates that recorded inner organoid cells are “OFF” cells (Cowan et al. 2020). Another study showed that the ON responses of RGCs can display a 25% increased response in spiking activity (Dorgau et al. 2019). In conclusion, the 150-day RO has been shown to generate synaptic connections in these two synaptic layers and to contain rudimentary functional synapses (Fig. 2).
Human RO as models for disease
RO as an inherited retina disease model
Human PSC-derived RO models are powerful tools for identifying disease mechanisms and for developing new therapies. To date, 24 studies have used patient-derived or gene-edited human PSC-derived ROs to model inherited retinal diseases (Table 2). Twelve of these studies include retinitis pigmentosa (RP) models, which involved 11 genes (Buskin et al. 2018; de Bruijn et al. 2020; Deng et al. 2018; Diakatou et al. 2021; Gao et al. 2020; Guo et al. 2019; Kallman et al. 2020; Lane et al. 2020; Quinn et al. 2019; Sharma et al. 2017; Zhang et al. 2020b; Zhang et al. 2021). ROs have also been used to identify the disease mechanisms of Leber’s congenital amaurosis (LCA)-related RPE65 (Li et al. 2019), CEP290 (Shimada et al. 2017), AIPL1 (Lukovic et al. 2020) and CRX (Kruczek et al. 2021). Other ocular diseases, such as glaucoma (VanderWall et al. 2020), macular telangiectasia type 2 (Gantner et al. 2019), microphthalmia (Eintracht et al. 2020), retinoblastoma (Deng et al. 2020; Liu et al. 2020; Saengwimol et al. 2020), Stargardt disease (Khan et al. 2020) and RS1-related X-linked juvenile retinoschisis as a model, moreover, a study on syndrome coronavirus 2 (SARS-CoV-2) demonstrated that SARS-CoV-2 can infect the retinal cells (Ahmad Mulyadi Lai et al. 2021). All these studies have been conducted in the last 4 years and have increased over time, which highlights the potential of RO as a model for retinal development and diseases. It is possible that future RO technology will enable many advances in retinal research.
RO in retinal disease therapy
As RO differentiation is convenient and stable, we can theoretically generate unlimited ROs, as well as transplantable retinal cells (Rabesandratana et al. 2020; Zhu et al. 2018). Additionally, the retinal cells in the RO have a similar stratified structure and cell-cell connection compared to the in vivo retina. In addition, ROs can either be produced from patient-derived induced PSCs (iPSCs) or from commercial human PSC lines and are more physiologically relevant than animal models. Therefore, ROs have become a reliable resource for cell therapy (Fischer et al. 2014; Gagliardi et al. 2018; Iraha et al. 2018; Kobayashi et al. 2018; McLelland et al. 2018; Singh et al. 2019; Xian et al. 2019; Xu et al. 2015; Zou et al. 2019) and drug screening (Khan et al. 2020; Liu et al. 2020), as well as a desired model for preclinical gene therapy(Garita-Hernandez et al. 2020; Gonzalez-Cordero et al. 2018; Tornabene et al. 2019; Volkner et al. 2021).
Cell therapy
Essentially, there are two strategies for RO-based cell therapy: transplantation of the RO specifically, of the retinal tissue (Iraha et al. 2018; McLelland et al. 2018; Singh et al. 2019), or transplantation of specific retinal cells (Gagliardi et al. 2018; Kobayashi et al. 2018; Zou et al. 2019). The major drawback of the former strategy involves integration issues. Although the transplanted retinal tissue can partially integrate with the host and display rescued retinal function, such as the light response, we can still clearly observe the boundaries between the extrinsic and intrinsic cells (Iraha et al. 2018; McLelland et al. 2018; Singh et al. 2019), which may lead to serious immune rejection without immunodeficient animal models (Iraha et al. 2018; Xian et al. 2019).
For specific retinal cell transplantation (except RPE transplantation, due to the fact that it is not directly derived from 3D-differentiated ROs), photoreceptors and retinal progenitor cells has been used for preclinical cell transplantation (Singh et al. 2020). The main problem of the retinal cell transplantation involves cell survival before and after transplantation. Before transplantation, the process of single-cell purification can lead to the deaths of retinal cells. Two-step immunopanning (Kobayashi et al. 2018) and specific cell surface markers for cell sorting (Gagliardi et al. 2018) have been shown to improve the cell survival rate (Table 1). Immune rejection also exists in this method; however, it is not as severe as tissue transplantation. Microglial activation and inflammation are crucial for the survivals of transplanted cells and host retinal cells during retinal degeneration, and the suppression of microglial activation can ameliorate the microenvironment to protect transplanted retinal cells from compromising cell viability (Zou et al. 2019). However, reactive microglia can also promote the formation of Müller-derived retinal progenitors (Fischer et al. 2014) and can facilitate retinal progenitor proliferation and differentiation into neuron-like cells (Xu et al. 2015). Therefore, modulating microglial activity is a potential approach to facilitate the success of retinal cell transplantations. Coculturing microglia with ROs and the subsequent transplantation of the microglia with retinal cells are worth performing in future studies.
Gene therapy
The success of AAV-mediated RPE65 gene augmentation therapy encourages the application and research on retinal diseases. The prerequisite for AAV-mediated gene therapy involves the efficient infections of specific target cells (Juttner et al. 2019) and long-lasting expressions (Garita-Hernandez et al. 2020). As an in vitro model with lamination structures and partial functions, ROs can be utilized for AAV efficiency (Gonzalez-Cordero et al. 2018; Tornabene et al. 2019; Volkner et al. 2021) and for expression time tests(Garita-Hernandez et al. 2020), which greatly enhanced transduction efficiency in the retinal cells. The promotor of AAV is the key for specifying the targeting cell. By using the human CRX promoter, a AAV2-CRX vector can precisely target a distinct apical lamina of the photoreceptor layer in the RO and function properly after infection (Kruczek et al. 2021). Thus, the effectiveness of AAV-mediated gene therapy will be significantly improved. In addition to the viral gene delivery system, the non-viral vectors based on cationic niosomes (Gallego et al. 2019; Mashal et al. 2019) and lipid nanoparticles (Patel et al. 2019) have been utilized in the rodents, the non-viral vector gene delivery system shows great potentials in therapeutic modality, for its low immunogenicity and high packing DNA size. Applying the non-viral vector to the retina organoids, is also worth trying.
The other gene therapy strategy involves gene editing of the PSC and subsequent differentiation into the RO. There have been four studies using this approach on patient-derived iPSCs (Buskin et al. 2018; Deng et al. 2018; Huang et al. 2019; Zhang et al. 2021). All of these studies proved that correct gene expression in the iPSCs can rescue the dysfunction caused by mutations in ROs. The difference among these studies includes the methods for gene editing, which involved double-strand break-based homology-directed repair in three researches (Buskin et al. 2018; Deng et al. 2018; Zhang et al. 2021) and targeted nucleotide alterations named base editing in one study (Huang et al. 2019). Although both methods exhibit off-target risks, with the accelerated progression of genome-editing technologies, it is possible that reductions in off-target editing activity (with the simultaneous maintenance of on-target editing efficiency) will be realized.
Conclusions
Retinal organoids provide optimal platforms for modeling retinal diseases and development. More importantly, via large-scale transcriptome analyses, we are able to address the similarities and differences between the human RO and the human retina to determine how well the present RO models are recapitulating the human retina (Collin et al. 2019; Cowan et al. 2020; Kim et al. 2019; Mao et al. 2019; Sridhar et al. 2020). Additionally, ROs can reproduce the main retinal cells with correct stratified layers and can generate functional synapses. Furthermore, RO methods can supply abundant cell resources for cell therapy and provide patient derived ROs for gene therapy. So far, more than 20 patient-derived RO disease models have been established, which will ultimately provide widespread benefits and promote the development of personalized medicine.
The progression from the initial 3D retinal organoid generation and characterization of retinal cells to their development into precise disease model agents has occurred at an accelerated pace. Before the development of ROs, transgenic mice were the main models for studies on retinal development and retinal diseases. In less than 10 years, numerous RO differentiation protocols, bioreactors, retina-on-chip and coculture systems have been developed to improve the efficiency and reality of ROs, which brings us closer to the efficient production of desired retinal organoids that are equipped with vascular systems and immune systems. With the rapid development of the RO system that can mimic the native retina, a promising and well-characterized model for retinal development and retinal disease will be established, which can significantly replace mouse models.
Availability of data and materials
Not applicable.
References
Achberger K, Probst C, Haderspeck J, Bolz S, Rogal J, Chuchuy J, et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife. 2019;8:e46188.
Aguirre G. Criteria for development of animal models of diseases of the eye. Am J Pathol. 1980;101:S187–96.
Ahmad Mulyadi Lai HI, Chou SJ, Chien Y, Tsai PH, Chien CS, Hsu CC, et al. Expression of Endogenous Angiotensin-Converting Enzyme 2 in Human Induced Pluripotent Stem Cell-Derived Retinal Organoids. Int J Mol Sci. 2021;22:1320.
Akhtar T, Xie H, Khan MI, Zhao H, Bao J, Zhang M, et al. Accelerated photoreceptor differentiation of hiPSC-derived retinal organoids by contact co-culture with retinal pigment epithelium. Stem Cell Res. 2019;39:101491.
Albert DM. Needs for animal models of human diseases of the eye: induced animal models of human ocular disease with particular consideration of ocular melanoma. Am J Pathol. 1980;101:S177–85.
Aparicio JG, Hopp H, Choi A, Mandayam Comar J, Liao VC, Harutyunyan N, et al. Temporal expression of CD184(CXCR4) and CD171(L1CAM) identifies distinct early developmental stages of human retinal ganglion cells in embryonic stem cell derived retina. Exp Eye Res. 2017;154:177–89.
Brooks MJ, Chen HY, Kelley RA, Mondal AK, Nagashima K, De Val N, et al. Improved retinal Organoid differentiation by modulating signaling pathways revealed by comparative Transcriptome analyses with development in vivo. Stem Cell Rep. 2019;13:891–905.
Buskin A, Zhu L, Chichagova V, Basu B, Mozaffari-Jovin S, Dolan D, et al. Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nat Commun. 2018;9:4234.
Capowski EE, Samimi K, Mayerl SJ, Phillips MJ, Pinilla I, Howden SE, et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development. 2019;146:dev171686.
Chavali VRM, Haider N, Rathi S, Vrathasha V, Alapati T, He J, et al. Dual SMAD inhibition and Wnt inhibition enable efficient and reproducible differentiations of induced pluripotent stem cells into retinal ganglion cells. Sci Rep-Uk. 2020;10:11828.
Chen TC, She PY, Chen DF, Lu JH, Yang CH, Huang DS, et al. Polybenzyl Glutamate Biocompatible Scaffold Promotes the Efficiency of Retinal Differentiation toward Retinal Ganglion Cell Lineage from Human-Induced Pluripotent Stem Cells. Int J Mol Sci. 2019;20:178.
Collin J, Queen R, Zerti D, Dorgau B, Hussain R, Coxhead J, et al. Deconstructing retinal Organoids: single cell RNA-Seq reveals the cellular components of human pluripotent stem cell-derived retina. Stem Cells. 2019;37:593–8.
Corral-Serrano JC, Lamers IJC, van Reeuwijk J, Duijkers L, Hoogendoorn ADM, Yildirim A, et al. PCARE and WASF3 regulate ciliary F-actin assembly that is required for the initiation of photoreceptor outer segment disk formation. P Natl Acad Sci USA. 2020;117:9922–31.
Cowan CS, Renner M, De Gennaro M, Gross-Scherf B, Goldblum D, Hou YY, et al. Cell types of the human retina and its Organoids at single-cell resolution. Cell. 2020;182:1623.
de Bruijn SE, Fiorentino A, Ottaviani D, Fanucchi S, Melo US, Corral-Serrano JC, et al. Structural variants create new topological-associated domains and ectopic retinal enhancer-gene contact in dominant retinitis Pigmentosa. Am J Hum Genet. 2020;107:802–14.
Deng WL, Gao ML, Lei XL, Lv JN, Zhao H, He KW, et al. Gene correction reverses Ciliopathy and photoreceptor loss in iPSC-derived retinal Organoids from retinitis Pigmentosa patients. Stem Cell Rep. 2018;10:1267–81.
Deng X, Iwagawa T, Fukushima M, Watanabe S. Characterization of human-induced pluripotent stem cells carrying homozygous RB1 gene deletion. Genes Cells. 2020;25:510–7.
Diakatou M, Dubois G, Erkilic N, Sanjurjo-Soriano C, Meunier I, Kalatzis V. Allele-specific knockout by CRISPR/Cas to treat autosomal dominant retinitis Pigmentosa caused by the G56R mutation in NR2E3. Int J Mol Sci. 2021;22:2607.
DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, et al. Accelerated and improved differentiation of retinal Organoids from pluripotent stem cells in Rotating-Wall vessel bioreactors. Stem Cell Rep. 2018;10:300–13.
Dorgau B, Felemban M, Hilgen G, Kiening M, Zerti D, Hunt NC, et al. Decellularised extracellular matrix-derived peptides from neural retina and retinal pigment epithelium enhance the expression of synaptic markers and light responsiveness of human pluripotent stem cell derived retinal organoids. Biomaterials. 2019;199:63–75.
Eintracht J, Toms M, Moosajee M. The Use of Induced Pluripotent Stem Cells as a Model for Developmental Eye Disorders. Front Cell Neurosci. 2020;14:265.
Eldred KC, Hadyniak SE, Hussey KA, Brenerman B, Zhang PW, Chamling X, et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science. 2018;362:eaau6348.
Fischer AJ, Zelinka C, Gallina D, Scott MA, Todd L. Reactive microglia and macrophage facilitate the formation of Muller glia-derived retinal progenitors. Glia. 2014;62:1608–28.
Fligor CM, Langer KB, Sridhar A, Ren Y, Shields PK, Edler MC, et al. Three-dimensional retinal Organoids facilitate the investigation of retinal ganglion cell development, Organization and Neurite Outgrowth from Human Pluripotent Stem Cells. Sci Rep-Uk. 2018;8:14520.
Fligor CM, Lavekar SS, Harkin J, Shields PK, VanderWall KB, Huang KC, et al. Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Rep. 2021;16:2228–41.
Freude KK, Saruhanian S, McCauley A, Paterson C, Odette M, Oostenink A, et al. Enrichment of retinal ganglion and Muller glia progenitors from retinal organoids derived from human induced pluripotent stem cells - possibilities and current limitations. World J Stem Cells. 2020;12:1171–83.
Gagliardi G, Ben M'Barek K, Chaffiol A, Slembrouck-Brec A, Conart JB, Nanteau C, et al. Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal Organoids. Stem Cell Rep. 2018;11:665–80.
Gallego I, Villate-Beitia I, Martinez-Navarrete G, Menendez M, Lopez-Mendez T, Soto-Sanchez C, et al. Non-viral vectors based on cationic niosomes and minicircle DNA technology enhance gene delivery efficiency for biomedical applications in retinal disorders. Nanomedicine. 2019;17:308–18.
Gantner ML, Eade K, Wallace M, Handzlik MK, Fallon R, Trombley J, et al. Serine and lipid metabolism in macular disease and peripheral neuropathy. N Engl J Med. 2019;381:1422–33.
Gao ML, Lei XL, Han F, He KW, Jin SQ, Zhang YY, et al. Patient-specific retinal Organoids recapitulate disease features of late-onset retinitis Pigmentosa. Front Cell Dev Biol. 2020;8:128.
Garita-Hernandez M, Chaffiol A, Guibbal L, Routet F, Khabou H, Riancho L, et al. Control of Microbial Opsin Expression in Stem Cell Derived Cones for Improved Outcomes in Cell Therapy. Front Cell Neurosci. 2021;15:648210.
Garita-Hernandez M, Guibbal L, Toualbi L, Routet F, Chaffiol A, Winckler C, et al. Optogenetic light sensors in human retinal Organoids. Front Neurosci-Switz. 2018;12:789.
Garita-Hernandez M, Routet F, Guibbal L, Khabou H, Toualbi L, Riancho L, et al. AAV-mediated gene delivery to 3D retinal Organoids derived from human induced pluripotent stem cells. Int J Mol Sci. 2020;21:994.
Ghareeb AE, Lako M, Steel DH. Coculture techniques for modeling retinal development and disease, and enabling regenerative medicine. Stem Cells Transl Med. 2020;9:1531–48.
Gonzalez-Cordero A, Goh D, Kruczek K, Naeem A, Fernando M, Kleine Holthaus SM, et al. Assessment of AAV vector tropisms for mouse and human pluripotent stem cell-derived RPE and photoreceptor cells. Hum Gene Ther. 2018;29:1124–39.
Guo YL, Wang PY, Ma JH, Cui ZK, Yu Q, Liu SW, et al. Modeling retinitis Pigmentosa: retinal Organoids generated from the iPSCs of a patient with the USH2A mutation show early developmental abnormalities. Front Cell Neurosci. 2019;13:361.
Hallam D, Hilgen G, Dorgau B, Zhu L, Yu M, Bojic S, et al. Human-induced pluripotent stem cells generate light responsive retinal Organoids with variable and nutrient-dependent efficiency. Stem Cells. 2018;36:1535–51.
Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–31.
Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6:402–20.
Huang KC, Wang ML, Chen SJ, Kuo JC, Wang WJ, Nhi Nguyen PN, et al. Morphological and molecular defects in human three-dimensional retinal Organoid model of X-linked juvenile Retinoschisis. Stem Cell Rep. 2019;13:906–23.
Iraha S, Tu HY, Yamasaki S, Kagawa T, Goto M, Takahashi R, et al. Establishment of Immunodeficient retinal degeneration model mice and functional maturation of human ESC-derived retinal sheets after transplantation. Stem Cell Rep. 2018;10:1059–74.
Jin ZB, Huang XF, Lv JN, Xiang L, Li DQ, Chen J, et al. SLC7A14 linked to autosomal recessive retinitis pigmentosa. Nat Commun. 2014;5:3517.
Juttner J, Szabo A, Gross-Scherf B, Morikawa RK, Rompani SB, Hantz P, et al. Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat Neurosci. 2019;22:1345–56.
Kallman A, Capowski EE, Wang J, Kaushik AM, Jansen AD, Edwards KL, et al. Investigating cone photoreceptor development using patient-derived NRL null retinal organoids. Commun Biol. 2020;3:82.
Keeler CE, Sutcliffe E, Chaffee EL. A description of the ontogenetic development of retinal action currents in the house mouse. Proc Natl Acad Sci U S A. 1928;14:811–5.
Khan KN, Mahroo OA, Khan RS, Mohamed MD, McKibbin M, Bird A, et al. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog Retin Eye Res. 2016;53:70–106.
Khan M, Arno G, Fakin A, Parfitt DA, Dhooge PPA, Albert S, et al. Detailed Phenotyping and therapeutic strategies for Intronic ABCA4 variants in Stargardt disease. Mol Ther Nucleic Acids. 2020;21:412–27.
Kim S, Lowe A, Dharmat R, Lee S, Owen LA, Wang J, et al. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids. Proc Natl Acad Sci U S A. 2019;116:10824–33.
Kobayashi W, Onishi A, Tu HY, Takihara Y, Matsumura M, Tsujimoto K, et al. Culture Systems of Dissociated Mouse and Human Pluripotent Stem Cell-Derived Retinal Ganglion Cells Purified by two-step Immunopanning. Invest Ophthalmol Vis Sci. 2018;59:776–87.
Kruczek K, Qu Z, Gentry J, Fadl BR, Gieser L, Hiriyanna S, et al. Gene therapy of dominant CRX-Leber congenital Amaurosis using patient stem cell-derived retinal Organoids. Stem Cell Rep. 2021;16:252–63.
Kruczek K, Swaroop A. Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cells. 2020;38:1206–15.
Kuwahara A, Ozone C, Nakano T, Saito K, Eiraku M, Sasai Y. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat Commun. 2015;6:6286.
Lane A, Jovanovic K, Shortall C, Ottaviani D, Panes AB, Schwarz N, et al. Modeling and rescue of RP2 retinitis Pigmentosa using iPSC-derived retinal Organoids. Stem Cell Rep. 2020;15:67–79.
Li G, Gao G, Wang P, Song X, Xu P, Xie B, et al. Generation and characterization of induced pluripotent stem cells and retinal Organoids from a Leber's congenital Amaurosis patient with novel RPE65 mutations. Front Mol Neurosci. 2019;12:212.
Liu H, Zhang Y, Zhang YY, Li YP, Hua ZQ, Zhang CJ, et al. Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proc Natl Acad Sci U S A. 2020;117:33628–38.
Lowe A, Harris R, Bhansali P, Cvekl A, Liu W. Intercellular adhesion-dependent cell survival and ROCK-regulated Actomyosin-driven forces mediate self-formation of a retinal Organoid. Stem Cell Rep. 2016;6:743–56.
Lukovic D, Artero Castro A, Kaya KD, Munezero D, Gieser L, Davo-Martinez C, et al. Retinal Organoids derived from hiPSCs of an AIPL1-LCA patient maintain Cytoarchitecture despite reduced levels of mutant AIPL1. Sci Rep. 2020;10:5426.
Luo Z, Zhong X, Li K, Xie B, Liu Y, Ye M, et al. An optimized system for effective derivation of three-dimensional retinal tissue via Wnt signaling regulation. Stem Cells. 2018;36:1709–22.
Mahato B, Kaya KD, Fan Y, Sumien N, Shetty RA, Zhang W, et al. Pharmacologic fibroblast reprogramming into photoreceptors restores vision. Nature. 2020;581:83.
Manafi N, Shokri F, Achberger K, Hirayama M, Mohammadi MH, Noorizadeh F, et al. Organoids and organ chips in ophthalmology. Ocul Surf. 2021;19:1–15.
Manian KV, Galloway CA, Dalvi S, Emanuel AA, Mereness JA, Black W, et al. 3D iPSC modeling of the retinal pigment epithelium-choriocapillaris complex identifies factors involved in the pathology of macular degeneration. Cell Stem Cell. 2021;28:846–62.
Mao X, An Q, Xi H, Yang XJ, Zhang X, Yuan S, et al. Single-cell RNA sequencing of hESC-derived 3D retinal Organoids reveals novel genes regulating RPC commitment in early human Retinogenesis. Stem Cell Rep. 2019;13:747–60.
Marshall JJ, Mason JO. Mouse vs man: Organoid models of brain development & disease. Brain Res. 2019;1724:146427.
Mashal M, Attia N, Martinez-Navarrete G, Soto-Sanchez C, Fernandez E, Grijalvo S, et al. Gene delivery to the rat retina by non-viral vectors based on chloroquine-containing cationic niosomes. J Control Release. 2019;304:181–90.
McLelland BT, Lin B, Mathur A, Aramant RB, Thomas BB, Nistor G, et al. Transplanted hESC-derived retina Organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Invest Ophthalmol Vis Sci. 2018;59:2586–603.
Mellough CB, Collin J, Khazim M, White K, Sernagor E, Steel DH, et al. IGF-1 signaling plays an important role in the formation of three-dimensional laminated neural retina and other ocular structures from human embryonic stem cells. Stem Cells. 2015;33:2416–30.
Mittal R, Woo FW, Castro CS, Cohen MA, Karanxha J, Mittal J, et al. Organ-on-chip models: implications in drug discovery and clinical applications. J Cell Physiol. 2019;234:8352–80.
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–85.
O'Brien JM, Marcus DM, Bernards R, Carpenter JL, Windle JJ, Mellon P, et al. A transgenic mouse model for trilateral retinoblastoma. Arch Ophthalmol. 1990;108:1145–51.
Ohlemacher SK, Langer KB, Fligor CM, Feder EM, Edler MC, Meyer JS. Advances in the differentiation of retinal ganglion cells from human pluripotent stem cells. Adv Exp Med Biol. 2019;1186:121–40.
Ohlemacher SK, Sridhar A, Xiao Y, Hochstetler AE, Sarfarazi M, Cummins TR, et al. Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous Neurodegeneration. Stem Cells. 2016;34:1553–62.
Olsson JE, Gordon JW, Pawlyk BS, Roof D, Hayes A, Molday RS, et al. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992;9:815–30.
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–79.
Ovando-Roche P, West EL, Branch MJ, Sampson RD, Fernando M, Munro P, et al. Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther. 2018;9:156.
Pan D, Xia XX, Zhou H, Jin SQ, Lu YY, Liu H, et al. COCO enhances the efficiency of photoreceptor precursor differentiation in early human embryonic stem cell-derived retinal organoids. Stem Cell Res Ther. 2020;11:366.
Patel S, Ryals RC, Weller KK, Pennesi ME, Sahay G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J Control Release. 2019;303:91–100.
Pereiro X, Miltner AM, La Torre A, Vecino E. Effects of Adult Muller Cells and Their Conditioned Media on the Survival of Stem Cell-Derived Retinal Ganglion Cells. Cells Basel. 2020;9:1759.
Quinn PM, Buck TM, Mulder AA, Ohonin C, Alves CH, Vos RM, et al. Human iPSC-derived retinas recapitulate the fetal CRB1 CRB2 complex formation and demonstrate that photoreceptors and Muller glia are targets of AAV5. Stem Cell Rep. 2019;12:906–19.
Rabesandratana O, Chaffiol A, Mialot A, Slembrouck-Brec A, Joffrois C, Nanteau C, et al. Generation of a Transplantable Population of Human iPSC-Derived Retinal Ganglion Cells. Front Cell Dev Biol. 2020;8:585675.
Ramamurthy V, Niemi GA, Reh TA, Hurley JB. Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 2004;101:13897–902.
Saengwimol D, Chittavanich P, Laosillapacharoen N, Srimongkol A, Chaitankar V, Rojanaporn D, et al. Silencing of the long noncoding RNA MYCNOS1 suppresses activity of MYCN-amplified retinoblastoma without RB1 mutation. Invest Ophth Vis Sci. 2020;61:8.
Santos-Ferreira T, Herbig M, Otto O, Carido M, Karl MO, Michalakis S, et al. Morpho-rheological fingerprinting of rod photoreceptors using real-time deformability Cytometry. Cytom Part A. 2019;95:1145–57.
Santos-Ferreira T, Volkner M, Borsch O, Haas J, Cimalla P, Vasudevan P, et al. Stem cell-derived photoreceptor transplants differentially integrate into mouse models of cone-rod dystrophy. Invest Ophth Vis Sci. 2016;57:3509–20.
Sarin S, Zuniga-Sanchez E, Kurmangaliyev YZ, Cousins H, Patel M, Hernandez J, et al. Role for Wnt signaling in retinal Neuropil development: analysis via RNA-Seq and in vivo somatic CRISPR mutagenesis. Neuron. 2018;98:109–126 e108.
Sharma TP, Wiley LA, Whitmore SS, Anfinson KR, Cranston CM, Oppedal DJ, et al. Patient-specific induced pluripotent stem cells to evaluate the pathophysiology of TRNT1-associated retinitis pigmentosa. Stem Cell Res. 2017;21:58–70.
Shimada H, Lu Q, Insinna-Kettenhofen C, Nagashima K, English MA, Semler EM, et al. In vitro modeling using Ciliopathy-patient-derived cells reveals distinct cilia dysfunctions caused by CEP290 mutations. Cell Rep. 2017;20:384–96.
Shinoe T, Kuribayashi H, Saya H, Seiki M, Aburatani H, Watanabe S. Identification of CD44 as a cell surface marker for Muller glia precursor cells. J Neurochem. 2010;115:1633–42.
Singh HP, Wang S, Stachelek K, Lee S, Reid MW, Thornton ME, et al. Developmental stage-specific proliferation and retinoblastoma genesis in RB-deficient human but not mouse cone precursors. Proc Natl Acad Sci U S A. 2018;115:E9391–400.
Singh MS, Park SS, Albini TA, Canto-Soler MV, Klassen H, MacLaren RE, et al. Retinal stem cell transplantation: balancing safety and potential. Prog Retin Eye Res. 2020;75:100779.
Singh RK, Occelli LM, Binette F, Petersen-Jones SM, Nasonkin IO. Transplantation of human embryonic stem cell-derived retinal tissue in the subretinal space of the cat eye. Stem Cells Dev. 2019;28:1151–66.
Sridhar A, Hoshino A, Finkbeiner CR, Chitsazan A, Dai L, Haugan AK, et al. Single-cell Transcriptomic comparison of human fetal retina, hPSC-derived retinal Organoids, and long-term retinal cultures. Cell Rep. 2020;30:1644.
Tanaka T, Yokoi T, Tamalu F, Watanabe S, Nishina S, Azuma N. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci Rep. 2015;5:8344.
Too LK, Gracie G, Hasic E, Iwakura JH, Cherepanoff S. Adult human retinal Muller glia display distinct peripheral and macular expression of CD117 and CD44 stem cell-associated proteins. Acta Histochem. 2017;119:142–9.
Tornabene P, Trapani I, Minopoli R, Centrulo M, Lupo M, de Simone S, et al. Intein-mediated protein trans-splicing expands adeno-associated virus transfer capacity in the retina. Sci Transl Med. 2019;11:eaav4523.
VanderWall KB, Huang KC, Pan Y, Lavekar SS, Fligor CM, Allsop AR, et al. Retinal ganglion cells with a Glaucoma OPTN(E50K) mutation exhibit neurodegenerative phenotypes when derived from three-dimensional retinal Organoids. Stem Cell Rep. 2020;15:52–66.
Veleri S, Lazar CH, Chang B, Sieving PA, Banin E, Swaroop A. Biology and therapy of inherited retinal degenerative disease: insights from mouse models. Dis Model Mech. 2015;8:109–29.
Volkner M, Pavlou M, Buning H, Michalakis S, Karl M. Optimized adeno-associated virus vectors for efficient transduction of human retinal organoids. Hum Gene Ther. 2021;32:694–706.
Volkner M, Zschatzsch M, Rostovskaya M, Overall RW, Busskamp V, Anastassiadis K, et al. Retinal Organoids from pluripotent stem cells efficiently recapitulate Retinogenesis. Stem Cell Rep. 2016;6:525–38.
Wang Z, Liu CH, Huang S, Chen J. Wnt signaling in vascular eye diseases. Prog Retin Eye Res. 2019;70:110–33.
Xian B, Luo Z, Li K, Li K, Tang M, Yang R, et al. Dexamethasone provides effective immunosuppression for improved survival of retinal Organoids after Epiretinal transplantation. Stem Cells Int. 2019;2019:7148032.
Xu Y, Balasubramaniam B, Copland DA, Liu J, Armitage MJ, Dick AD. Activated adult microglia influence retinal progenitor cell proliferation and differentiation toward recoverin-expressing neuron-like cells in a co-culture model. Graefes Arch Clin Exp Ophthalmol. 2015;253:1085–96.
Zerti D, Dorgau B, Felemban M, Ghareeb AE, Yu M, Ding Y, et al. Developing a simple method to enhance the generation of cone and rod photoreceptors in pluripotent stem cell-derived retinal organoids. Stem Cells. 2020;38:45–51.
Zerti D, Molina MM, Dorgau B, Mearns S, Bauer R, Al-Aama J, et al. IGFBPs mediate IGF-1's functions in retinal lamination and photoreceptor development during pluripotent stem cell differentiation to retinal organoids. Stem Cells. 2021;39:458–66.
Zhang X, Serb JM, Greenlee MH. Mouse retinal development: a dark horse model for systems biology research. Bioinform Biol Insights. 2011;5:99–113.
Zhang X, Tenerelli K, Wu SQ, Xia X, Yokota S, Sun C, et al. Cell transplantation of retinal ganglion cells derived from hESCs. Restor Neurol Neuros. 2020a;38:131–40.
Zhang X, Thompson JA, Zhang D, Charng J, Arunachalam S, McLaren TL, et al. Characterization of CRB1 splicing in retinal organoids derived from a patient with adult-onset rod-cone dystrophy caused by the c.1892A>G and c.2548G>a variants. Mol Genet Genomic Med. 2020b;8:e1489.
Zhang X, Zhang D, Thompson JA, Chen SC, Huang Z, Jennings L, et al. Gene correction of the CLN3 c.175G>a variant in patient-derived induced pluripotent stem cells prevents pathological changes in retinal organoids. Mol Genet Genomic Med. 2021;9:e1601.
Zhang XH, Jin ZB. Patient iPSC-derived retinal organoids: Observable retinal diseases in-a-dish. Histol Histopathol. 2021;35:18307.
Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047.
Zhou S, Flamier A, Abdouh M, Tetreault N, Barabino A, Wadhwa S, et al. Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFbeta and Wnt signaling. Development. 2015;142:3294–306.
Zhu J, Reynolds J, Garcia T, Cifuentes H, Chew S, Zeng X, et al. Generation of transplantable retinal photoreceptors from a current good manufacturing practice-manufactured human induced pluripotent stem cell line. Stem Cells Transl Med. 2018;7:210–9.
Zou T, Gao L, Zeng Y, Li Q, Li Y, Chen S, et al. Organoid-derived C-kit(+)/SSEA4(−) human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents. Nat Commun. 2019;10:1205.
Acknowledgments
We thank for all the help from the 502 team.
Funding
This work is partly supported by the Beijing Municipal Natural Science Foundation (Z200014) and National Key R&D Program of China (2017YFA0105300).
Author information
Authors and Affiliations
Contributions
XZ collected literate and wrote the manuscript, XZ, WW drew the figures and tables, ZBJ outlined the contents and revise the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, X., Wang, W. & Jin, ZB. Retinal organoids as models for development and diseases. Cell Regen 10, 33 (2021). https://doi.org/10.1186/s13619-021-00097-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13619-021-00097-1