Abstract
Premature ovarian failure (POF) affects 1% of women under 40, leading to infertility. The clinical symptoms of the POF include hypoestrogenism, lack of mature follicles, hypergonadotropinism, and amenorrhea. POF can be caused due to genetic defects, autoimmune illnesses, and environmental factors. The conventional treatment of POF remains a limited success rate. Therefore, an innovative treatment strategy like the regeneration of premature ovaries by using human umbilical cord mesenchymal stem cells (hUC-MSCs) can be a choice. To summarize all the theoretical frameworks for additional research and clinical trials, this review article highlights all the results, pros, and cons of the hUC-MSCs used to treat POF. So far, the data shows promising results regarding the treatment of POF using hUC-MSCs. Several properties like relatively low immunogenicity, multipotency, multiple origins, affordability, convenience in production, high efficacy, and donor/recipient friendliness make hUC-MSCs a good choice for treating basic POF. It has been reported that hUC-MSCs impact and enhance all stages of injured tissue regeneration by concurrently stimulating numerous pathways in a paracrine manner, which are involved in the control of ovarian fibrosis, angiogenesis, immune system modulation, and apoptosis. Furthermore, some studies demonstrated that stem cell treatment could lead to hormone-level restoration, follicular activation, and functional restoration of the ovaries. Therefore, all the results in hand regarding the use of hUC-MSCs for the treatment of POF encourage researchers for further clinical trials, which will overcome the ongoing challenges and make this treatment strategy applicable to the clinic in the near future.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
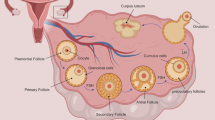
The ovaries are complex and critical reproductive organs in the female body. Multiple factors can affect the function of ovaries leading to infertility in females. The outside layer of the ovaries contains unique structures known as follicles. These follicles produce an oocyte (immature egg), which becomes mature into a fertilizable egg by a process known as folliculogenesis. Ovarian follicles include three categories of cells: oocytes, theca and granulosa. Follicle growth and development depends on the follicle-stimulating hormone (FSH) and luteinizing hormone (LH) receptors, which are found in the granulosa and theca cells. Folliculogenesis is a well-planned and regulated process. The process involves the development of primordial follicles into primary, preantral, and ultimately antral follicles. Ovulation happens after this stage (Fig. 1) [1]. The number of primordial follicles is restricted during a woman's reproductive life. Females are said to have entered reproductive senescence or menopause when their reserve is depleted. There are around 6 to 7 million germ cells in a female fetus. Approximately 400,000 to 500,000 primordial follicles persist by the time a girl enters adolescence. Approximately 1000 follicles each month are lost after menarche [2, 3].
Around age 50, there are only around 1000 follicles left after the number of follicles reduces to about 25,000 after age 37, and the rate of follicular loss increases. Thus, throughout a woman's reproductive life, only about 400 follicles will develop and ovulate, with the large bulk never doing so [4]. The ovaries can stop functioning due to various reproductive abnormalities that affect the ovaries causing discomfort, irregular menstrual periods, urinary problems, infertility, and pregnancy failure. Among these, Premature Ovarian Failure (POF)/Primary Ovarian Insufficiency (POI), Polycystic Ovary Syndrome (PCOS), Asherman Syndrome, Endometriosis, and Preeclampsia are the most frequent female reproductive disorders [5, 6]. Premature ovarian failure (POF), aka (POI) Primary ovarian insufficiency or early menopause, is a puzzling and complex condition. POF affects one in every 250 women under 35 years and one in every 100 women under 40 years [7, 8]. POF has significant health implications for women. The climacteric syndrome (a group of symptoms brought on by a drop in ovarian hormone levels) is the primary source of short-term consequences comparable to spontaneous menopause. POF diminishes the likelihood of a natural pregnancy significantly. Urogenital atrophy is also caused by a lack of estrogen (Lower estrogen levels in these tissues, alterations in the vagina and urethra occur). Vaginal dryness, discomfort, and itching are the most prevalent urogenital symptoms. Sexual function is further hampered by urogenital atrophy and hypoestrogenism. A decline in bone mineral density (BMD) is a hazard to POF patients. In addition to experiencing psychological anguish, investigations on POF women have found that they have a higher chance of developing neurodegenerative disorders. Generally, POF women's life span is decreased due to cardiovascular illness, osteoporosis and sexual dysfunction [9, 10]. Hypergonadotropinism and amenorrhea are also among the main POF characteristics that lead to premenopausal syndrome and female infertility [11].
Pathophysiology of POF
Two histological forms of early ovarian failure have been identified. In the first type, the ovarian follicles are entirely depleted, but in type 2, the ovary retains follicular features. According to Nelson et al. [8] the essential processes in POF are follicle depletion and follicular dysfunction. The quality of the oocytes and follicular pool can be affected by genetic, paracrine, endocrine, mitochondrial dysfunction, and metabolic variables, yet the origin of POF is unknown [12]. FSH (follicle-stimulating hormone) levels in the early follicular phase, estradiol, inhibin B, and FSH/luteinizing hormone (LH) levels are utilized to make the diagnosis [13]. A high FSH level is a clear indicator of ovarian failure. In addition to these, more hormones can be measured, which include stimulating thyroid hormone (TSH), prolactin (PRL) and anti-Müllerian hormone (AMH) [14]. Two types of inheritance patterns for POF exist: sporadic and familial [15]. Among the leading causes of POF are Iatrogenic factors, such as pelvic surgery and chemotherapy, environmental factors, such as viral infections, radiation, and toxins, autoimmune diseases with anti-ovarian antibodies causing ovarian damage, and genetic changes, such as point mutation, chromosome imbalances involving the X chromosome or autosomes (Fig. 2) [16]. Over 50% of POF cases are still idiopathic despite improvements in medicine, and they might manifest in random or familial forms [17]. Genetics may directly cause the disease or merely predispose a person to it. Regarding POF, certain elements may be found in around 20–25% of instances [18]. POF is inherited in 4–31% of cases, with an X-chromosome aberration playing a crucial part in the condition [15].
The pathogenic factors, leading causes (Iatrogenic factors, environmental stressors, genetic and autoimmune factors), and treatment options of POF (hormone replacement therapy, androgen, counseling, synthesized bioidentical hormones, Dehydroepiandrosterone, donated oocytes, exercise and diet and stem cell treatment) along with sources of mesenchymal stem cells (adipose tissue, bone marrow, dental tissues, peripheral blood, placenta/umbilical cord(UC), dermal tissue)
Conventional Treatment of POF
Several treatments have been proposed owing to the complexities of POF. However, none of them has shown to be sufficiently successful in serving as a reliable first-line alternative. Treatments for POF include hormone replacement therapy, androgen, counseling, synthesized bioidentical hormones, Dehydroepiandrosterone, donated oocytes, exercise and diet, and stem cell treatment (Fig. 2). There are two different forms of sex-steroid replacement (SSR): physiological sex-steroid replacement (pSSR) and standard sex-steroid replacement (sSSR). While pSSR is used to control hormone levels in aberrant ovarian function, sSSR has improved symptoms in postmenopausal women [19]. It has been suggested that Dehydroepiandrosterone (DHEA) is a different hormonal therapy for enhancing ovarian function. Count of Antral follicles considerably increased after 16 weeks of treatment in a recent clinical trial studying the impact of DHEA on POF patients by an evaluation of ovarian response indicators, while AMH or FSH levels were not significantly impacted [20]. Ovarian theca cells and the zona reticularis of the adrenal cortex are the sources of DHEA. Almost half of the androgens are made from DHEA in tissues, created by conversion from cholesterol and estrogens. Therefore, it has a vital role in the synthesis of Testosterone (T), Estradiol (E2), and finally Estriol (E2) within peripheral tissues. DHEA is a crucial prohormone in ovarian follicular steroidogenesis because of this. Patients with POF prescribed DHEA may increase their odds of getting pregnant and reduce their risk of miscarriage [19].
The use of androgen supplementation, especially testosterone, has been studied to enhance general and sexual health [21]. Due to assertions that compounded bioidentical hormone (CBH) is a safer, more effective alternative treatment option with fewer side effects than other forms of therapy, its use has significantly expanded. Best and Triest, two popular CBH formulations, comprise of 80% estriol and 20% estradiol by weight and 10% estradiol, 80% estriol and 10% estrone, and by weight respectively [22]. Even though methods for preserving fertility for POF patients are still being developed, donor oocytes that are cryopreserved should be considered, IVF treatments using fresh donor eggs have historically had a high cumulative pregnancy rate (88%) after four rounds. In addition to fresh and new ovum donation, the utilization of cryopreserved donor oocytes is now possible thanks to the invention of vitrification, a fast-freeze process [23].
Risks Associated with Conventional Treatments for POF
POF has a complex etiology, mainly with autosomal genetic abnormalities, X-chromosome abnormalities, immunological disorders, and enzyme deficiencies. In young individuals, the POF caused by chemotherapy medications is more evident [24]. Hormone replacement therapy (HRT) is the most prevalent POF treatment. The role of HRT in boosting fertility, on the other hand, is still debatable. Alternative therapies should be used to lessen the symptoms and risks associated with POF, as HRT is regarded as dangerous in women who have a history of ovarian cancer or breast cancer; it also raises the risk of blood clots, cancer, strokes, and other complications [25]. Egg donation is the last and most hopeful option for most POF women. However, donation of egg supplies is limited, and patients who receive these eggs will not be able to produce biological children of their own. As a result, specialists are on the lookout for more effective and innovative POF treatments. Scientists are turning to alternate therapies, such as stem cell therapy, to treat POF and other kinds of infertility due to adverse effects connected with HRT therapy used to treat POF and different types of infertility [26]. Mesenchymal Stem Cells transplantation appears to be a potential therapy option.
Stem Cell Therapy for POF Treatment
Recently, SC therapy has been proposed as a potential substitute for treating several illnesses and has been classified as regenerative medicine. Stem cells can differentiate and self-renew to repair and restore damaged tissues or cells [27, 28]. Stem cells are unspecialized, undifferentiated cells found in stages of embryo, fetal, and adult life. Stem cells may be found in many parts of the human body and are classified based on their origin like skin and adipose, umbilical cord, amniotic fluid, placenta, and bone marrow [29]. Some of the main stem cell types like ESCs: embryonic stem cells; MSCs: mesenchymal stem cells; SSCs: spermatogonial stem cells; iPSCs: induced pluripotent stem cells have all been used in stem cell-based treatment for infertility [5, 30]. Stem cell treatment for the possible generation of ovules in women can be performed in POF patients. Several kinds of stem cells usage for the treatment of POF has been documented in recent research [25, 29,30,31]. The promise of a long-term replacement for damaged oocytes has prompted many to explore stem cell (SC) treatment for infertility. These transplanted SCs may establish themselves inside the ovarian tissue and restore ovarian function, as seen in a chemotherapy-induced POF mouse model [32]. For efficient ovarian rejuvenation (stimulation of follicles in the early stages), many stem cell infusion techniques, including the administration of SCs into the ovaries, laparoscopic ovarian injection, infusion through the ovarian artery (intra-arterial catheterism), TVUS-guided ovarian injection, or direct ovarian infusion/injection are used. With the stimulation of dormant follicles, these procedures may allow for a partial reversal of the ovary's aging process. Even with standard drugs, these follicles would otherwise remain in the ovary without developing [32, 33]. For women with POF who want to get pregnant, stem cell therapy is a last-resort therapeutic option. SC therapy has shown encouraging outcomes thus far, with spontaneous pregnancies occurring in women with a poor ovarian reserve who had bone marrow Stem Cell therapy [34]. Extensive investigations on SCs capable of producing oocytes have been carried out in mice and humans. These findings give a reason for optimism in developing novel POF/POI therapies. By increasing the number of primordial follicles, decreasing granulosa cell (GC) mortality, and restoring ovary sex hormone activity, SCs from diverse sources may aid and support the restoration of ovarian function [34, 35].
Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells are multipotent mesenchymal stromal cells extracted from stromal tissues and possess plastic adhesion, self-renewal, and multi-lineage differentiation capabilities [36]. Many experts believe that transplanting mesenchymal stem cells (MSCs) is the most effective and successful way of cell therapy. These cells impact and enhance all stages of injured tissue regeneration by concurrently stimulating numerous pathways (trophic, paracrine, immunological modulation, and differentiation) [37]. MSCs originate from a variety of locations across the human body, including the bone marrow, amniotic membrane, amniotic fluid,, dental tissues, endometrium,, menstrual blood, limb buds, peripheral blood, fetal membrane and placenta, skin and foreskin, salivary gland, Wharton's jelly, sub-amniotic umbilical cord lining membrane, and synovial fluid (Fig. 2) [33, 38, 39]. MSCs provide therapeutic advantages via direct differentiation and paracrine actions, the latter of which is now regarded to be the most critical therapeutic mechanism. According to research, MSCs appear to have a more substantial influence on cell signaling than their natural regeneration abilities. Cell signaling has also been linked to regenerating and repairing dysfunctional cells [39]. Mesenchymal stem cells (MSCs) have been demonstrated in several human trials to restore ovarian function and provide a healthy treatment for women's infertility [35, 40,41,42]. MSCs extracted from various tissues and organs, including placenta, bone marrow, adipose tissue and endometrium, increased follicle development and improved development and quality of oocyte, ovulation, and fertility as therapies for POF [5, 43]. MSCs have homing capabilities, allowing them to accumulate spontaneously at the site of damage. As a result, MSCs migrate and attach to the wounded ovary, and multiply in response to numerous hormones and growth factors. According to these studies, MSCs boost ovarian function and aid in functional ovarian recovery. It is unknown whether MSCs differentiate into oocytes or assist stromal or follicular cells after migrating to the ovary to produce this impact [5, 35, 39,40,41,42].
Human Umbilical Cord as a Source of Human Umbilical Cord Mesenchymal Stem Cells (hUC-MSCs)
The human umbilical cord is a good source of MSCs. Unlike bone marrow stem cells, MSCs from the human umbilical cord are collected more quickly and painlessly. MSCs have also been found in the vascular tissue and Wharton jelly of the umbilical cord [44]. These cells exhibit the same triple activity as bone marrow cells in tissue repair, immune response regulation, and anti-cancer capabilities. Other pluripotent or embryonic stem cells can use hUC-MSCs as a source of nutrition [45,46,47,48]. UC-MSCs can be obtained in an ethically acceptable and non-invasive manner and large numbers of UC-MSCs can be produced following expansion. Umbilical cord (UC) contains one umbilical vein (UCV) and two umbilical arteries (UCAs), both embedded within Wharton’s jelly (WJ) which is a specific mucous connective tissue covered by amniotic epithelium (Fig. 3).
Different compartments of the umbilical cord tissue. One umbilical vein (UCV) and two umbilical arteries (UCAs) are implanted in Wharton's jelly (WJ) in the umbilical cord (UC) to form the structure of the umbilical cord (UC). The amniotic epithelium encloses mucous connective tissue. UC-Umbilical cord
hUC-MSCs can be derived from the whitish flesh of the umbilical cord, known as Wharton’s jelly (WJ-MSCs), umbilical cord blood (UC-MSCs) [49, 50] or human umbilical cord perivascular stem cells (HUCPVC) [51,52,53]. Even though UC blood (UCB) and the umbilical cord (UC) are commonly discarded as medical waste after delivery, they offer a rich source of stem cells. At least three types of stem cells, including endothelial progenitor cells, mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs), have been identified from UCB. MSCs have been isolated from numerous umbilical cord compartments. MSCs have been separated from umbilical cord blood, inside Wharton's jelly and umbilical vein subendothelium: the perivascular zone, the subamnion and the intervascular site [54]. The benefits of UC–MSCs over other MSC sources are non-invasive retrieval, off-the-shelf usage, and high abundance [55].
Advantages of hUC-MSCs
The umbilical cord is routinely discarded as medical waste after birth; therefore, it rules out ethical concerns about their access as the procedure is non-invasive. UC-MSCs, like MSCs from other sources, have a unique ability to self-renew while preserving their multipotency (the ability to develop into osteocytes, adipocytes, chondrocytes, hepatocytes, and neurons) [53]. Stem cells increase ovarian function through paracrine actions rather than developing into particular cells [56]. Different growth factors, cytokines, signaling lipids, regulatory miRNAs and messenger RNAs (mRNAs), are all found in the vesicles released by mesenchymal stem cells (MSCs). Cellular signaling, Cell-to-cell communication, and changes in tissue or cell metabolism are all affected by these variables [57]. The immunomodulatory characteristics of UC-MSCs have also grabbed people’s interest. UC-MSCs are now highlighted as a potentially adaptable regenerative medicine and immunotherapy strategy [53]. UCMSCs have few specific properties that they maintain namely, quick self-renewal, low risk of tumor, low chance of immune rejection, less ethical concerns, non-invasive and painless collection, and easy production in bulk.
Paracrine Effects of hUC-MSCs
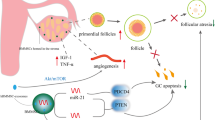
hUC-MSCs have a high cytokine secretion profile, allowing them to proliferate faster, differentiate more effectively, and have low immunogenicity. Interleukins (IL), colony-stimulating factors (CSF), tumor necrosis factor (TNF), interferons (IFN), chemokines and TGF are among them. These cytokines are involved in homing, anti-inflammatory, migration, anti- pro-angiogenesis, and apoptosis pathways in UCMSCs. It has been demonstrated in several investigations that these UCMSCs can repair compromised ovarian function by producing cytokines and other factors involved in proliferation and tissue formation [58]. Here is a proposed explanation of the fundamental mechanism of action that stem cells are thought to have (Fig. 4). It was reported that the ovaries treated with hUC-MSCs showed that vascularization in the ovarian niche was increased in the cyclic healing process and may be helpful for ovarian recovery in the context of angiogenesis. In vivo and in vitro, factors like (HGF) hepatocyte growth factor, (VEGF) Vascular Endothelial Growth Factor, insulin-like growth factor-1(IGF-1) and (FGF-2) Fibroblast Growth Factor-2 generated by these cells have been reported to induce arteriogenesis [59]. When VEGF and HGF are combined, vascular diameter is enhanced. HGF helps with vascular area expansion, whereas VEGF enhances the area, length, and several branch points of the induced vessels [60].
Proposed mechanisms of ovarian damage treatment using stem cells. The flow diagram represents how premature ovarian failure is treated by injecting MSCs through various methods and as a result MSCs act through paracrine signaling and are involved in the control of ovarian fibrosis, angiogenesis, immune system modulation, and apoptosis. Premature Ovarian Failure (POF), Mesenchymal Stem Cells (MSCs), Vascular Endothelial Growth Factor (VEGF), Fibroblast Growth Factor 2 (FGF2), Fibroblast Growth Factor (FGF), Hepatocyte Growth Factor (HGF), Insulin-Like Growth Factor Factor 1 (IGF-1), Anti-Müllerian hormone (AMH), Follicle-stimulating hormone (FSH).
Immunosuppressive Effects of hUC-MSCs
hUC-MSCs are an abundant source of MSCs that display stem cell-specific markers and can be differentiated into various types of mesodermal cell for immune response modulation and tissue repair. The poor immunogenic qualities are related to decreased production of MHC class I and class II proteins, which are required for adaptive immunity. MSCs interact with various immune cells; in vitro, they are poor stimulators of allogeneic T-cell responses [61]. The T-cell receptor recognizes MHC molecules in combination with an antigen that is on the surface of an (APC) antigen-presenting cell in the first signal [62, 63]. Following that, T-cell activation requires the CD28 on the T cell with CD86 or CD80 (B7 superfamily) interaction on the APC through a co-stimulatory signal. MSCs have low quantities of MHC-I molecules on their surface, but none of the MHC-II or co-stimulatory molecules like CD80, CD86, or CD40. Because MSC lack MHC class II, they can avoid being recognized by alloreactive CD4 + T cells. It is also worth noting that co-stimulatory chemicals are not present. Any remaining T cell receptor contact on Th cells will result in anergy and tolerance rather than an allogeneic response [64, 65]. MSCs have been shown to exhibit immunoregulatory capabilities in the ovarian niche by controlling the populations of macrophages, cytokines and regulatory T lymphocytes. MSCs interact with innate and adaptive immune systems immune cells to display immunoregulatory properties [60]. MSCs control T-cell growth and function [66]. MSCs only carry out immunoregulatory functions in the restricted target tissue; they have no systemic impact. Therefore, they do not inhibit the immune system systemically.
Furthermore, the hUC-MSCs were reported to restore ovarian function in POF mice via modulating the ratio of (Th1/Th2) T-helper-1 and T-helper-2 cytokines and the number of (NK) natural killer cells [67]. Moreover, the ovarian function is also improved through regulating cytokines like TGF-β, Treg cells, and interferon-g (IFN-g) [68]. By releasing hormones, cytokines, inhibiting apoptosis-related genes and upregulating proliferation-related genes, MSCs can decrease GC death and enhance GC proliferation [69]. Wang et al. [70] showed that the transplantation of MSCs might help in the restoration of ovarian function by upregulating and FSHR and AMH expression in POF mice's GCs, inhibiting GC apoptosis and follicular atresia. Another study by Fu et al. [25] showed that by releasing VEGF, IGF-1, HGF and upregulating Bcl-2 expression, BMSC transplantation might minimize GC apoptosis and enhance ovarian function. Ovarian cell proliferation and apoptosis rates are linked to ovarian function hence controlling MSC efficacy in POF therapy. A drop in collagen levels following the transplantation of MSCs has also been seen in connection to the reduction of fibrosis, indicating that the mechanism of action of these cells in the healing of ovarian injury may also entail an antifibrotic impact [71]. In fact, fibroblast proliferation may be inhibited and extracellular matrix deposition levels may be reduced by MSCs [72]. Also, MSCs have an immune-regulating impact, making them a prospective therapy option for immune-mediated POF. As a result, more comprehensive processes are required to boost therapeutic efficacy of MSCs and provide a foundation for their clinical application [25].
Pre-Clinical Trials for the Treatment of POF using hUC-MSCs
Although there are few studies on the usage of UCMSC in POF therapy, it has been shown that UCMSC transplantation may be able to restore ovarian function via the paracrine pathway. According to the findings, UCMSC expressed more proteins engaged in the epigenetic pathway, transcription pathway, DNA modification, cell signaling, and protein modification pathway. UCMSCs can swiftly heal the ovarian injury by stimulating the development of granulosa cells through these pathways. Some ovarian stem cell function proteins, such as Ly6a (Sca-1) and AKR1C18 [73,74,75], can be increased by UCMSCs. UCMSCs can release powerful combinations of trophic substances that alter the environment's molecular composition and elicit reactions from resident cells. Mesenchymal Stem Cells (MSCs) have been reported for their ability to heal damaged ovaries caused by chemotherapy [76]. UCMSCs can elude immunological reactivity even in allogeneic transplants because of lower expression of human leukocyte antigen (MHC-I) major histocompatibility complex I and lack of MHC-II molecules indicating that transplanting UCMSCs causes minimal to no immunological rejection [77].
Research also demonstrated that stem cells can prevent apoptosis by secreting stanniocalcin-1 and other paracrine substances [78,79,80]. Some pre-clinical studies indicate that hUC-MSCs were reported to restore ovarian function in POF mice via modulating the ratio of (Th1/Th2) T-helper-1 and T-helper-2 cytokines and the number of natural killer NK cells [67] and through regulating TGF-β, Treg cells, and (IFN-g) interferon-g cytokines [68]. Angiogenic growth factors that MSCs may release include TGF-, hepatocyte growth factor (HGF), (VEGF) vascular endothelial growth factor, and placental growth factor, and the protective effect of transplanted UCMSCs is mediated by factors that play a role in the restoration ovarian function by the secretion of NGF and TrkA to inhibit GC apoptosis (PGF) [81]. MSCs can increase GC proliferation and decrease GC death by producing cytokines and hormones, upregulating proliferation, and inhibiting apoptosis-related genes [69]. MSC transplantation restored ovarian function by upregulating AMH and FSHR expression in POF mice's GCs, inhibiting GC apoptosis and follicular atresia by releasing VEGF, HGF, and IGF-1 upregulating Bcl-2 expression [82].
A recent study on the POF rat model demonstrated that the indicators underlying ovarian function were restored by UCMSC transplantation through the TGF-β1/Smad3, Hippo, AMPK/mTOR and PI3K/Ak signaling pathways; UCMSCs could influence the development of ovaries, growth of follicles, cell proliferation and differentiation of granulosa [71, 83]. The PI3K pathway was shown to be activated by human UCMSCs when they were used for the restoration of ovarian function. This lowered monosaccharide content and enhanced lipid metabolism. After further investigation on a complete signaling pathway analysis of the molecular processes of repairment and damage on the injection of UCMSCs to rats with POF models, this work may provide a foundation for the therapeutic application of UCMSC to treat POF [84]. Another report on ovarian function restoration demonstrated that hUC-MSC transplantation could have a positive and preventative effect on rats using the POF model. (Deng et al., 2021)It was also seen that UC-MSCs significantly enhanced the function of ovaries in chemotherapy-induced POF by inhibiting apoptosis by reducing inflammation via activation of AKT and P38 pathways. These hUC-MSCs were injected into POF rats' ovaries, reducing sexual cycle dysfunction, enhancing serum hormone expression, and restoring ovarian function [85] (Deng et al., 2021). Overall ovarian cell proliferation and apoptosis rates are linked to ovarian function thereby controlling MSC efficacy in POF therapy. Also, MSCs have an immune-regulating impact, making them a prospective therapy option for immune-mediated POF. However an evaluation of more comprehensive processes are needed to increase the therapeutic effectiveness of MSCs and provide a foundation for their clinical application field.
Clinical Trials Regarding the Treatment of POF using hUC-MSCs
Numerous clinical research has been conducted to address the issues primarily related to pregnancy since POF has significant health implications for women. The primary outcomes of the disease have also been analyzed, such as the size of the ovaries; Serum hormone levels such as Anti-Müllerian hormone (AMH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol (E2). Secondary outcomes were also studied in clinical studies, such as the number of antral follicle counts (AFC) and dominant follicles (DFC) every month; the number of babies born and pregnancies; the quantity of harvested oocytes, matured oocytes, high-quality embryos, the percentages of clinical pregnancy, miscarriages, or live birth for ICSI /IVF cycles; as well as the frequency of unfavorable incidents such temperature, rash, vaginal bleeding, headaches, infectious diseases, abnormal liver functions, neoplasms, and aberrant renal function were also studied [86]. The first clinical studies were conducted on people using MSCs from bone marrow (BM) using SCs, through iliac crest aspiration for cell collection, Stem cell isolation, and invitro culture methods [72]. A stem cell therapy resulting in the live birth of a 2.7 kg female baby by a 45-year-old perimenopausal lady was reported by Gupta et al. [34]. Positive results with a similar technique, with 10 POF younger women, showed a restoration of menstruation in two patients and a pregnancy with live birth [87]. Similar to another study in 30 POF women (18–40 years old) resulted in one pregnancy and improved symptoms in fields [88].
According to research, BMDSC boosts ovarian vascularization, stromal cell proliferation, and cell apoptosis to enhance human and mouse follicular growth [89]. Based on this knowledge, a team created future pilot research on 17 POF women to examine the effects of autologous stem cell ovarian transplant (ASCOT) on ovarian reserve. As a result, six pregnancies and three different newborns were accomplished, and 81.3 percent of women had improved ovarian function biomarkers (AMH and AFC) [90]. According to the findings by Liu et al. [91], ASCOT may have enhanced the proliferation of preexisting follicles by mediating the presence of certain factors released by stem cells, namely FGF-2 and THSP-1, during aphaeresis [92]. It is crucial to note that numerous Randomized Controlled Trials (RCT) involving POF women are testing multiple additional stem cell origins across the globe. There are now eleven clinical studies using MSCs as a treatment for POI/POF patients globally, according to data from ClinicalTrials.gov. Out of these eleven studies, five include transplanting Human Umbilical Cord MSCs. Clinical trials on the transplantation of UCMSCs into POI/POF patients are still ongoing, and more research is still needed to determine if UCMSCs are effective in treating POI/POF. A Clinical Study titled “Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Premature Ovarian Insufficiency case studies” was submitted by Li-jun Ding, Nanjing University, in April 2022 (NCT05308342) (https://www.clinicaltrials.gov/). This was an interventional, single-center, randomized, controlled prospective study. To determine the efficiency and safety of hUC-MSC in the treatment of patients (Married Females, 20 years old ≤ age < 40 years old, having an average diameter of each ovary > 10 mm; with premature ovarian failure were enrolled and treated with hormone replacement combined with transplantation of hUC-MSC into ovaries. UC-MSCs were injected into the ovary of patients under transvaginal ultrasonographic (TVUS)-guidance by medical physicians. This ongoing study provides promising templates for future similar studies. However, most of them are still in progress. Thus, no outcomes have been announced (Table 1).
A study suggested that UCMSC's effects on reproduction may be caused by an increase in ovarian angiogenesis, which encourages granulosa cell proliferation and the creation and development of follicles. Four patients became pregnant following in vitro fertilization and embryo transfer using the UCMSC therapy (intraovarian injections of UCMSCs once to three times), and their offspring developed usually. This treatment successfully restored ovarian function in 61 women without causing severe adverse events [86].
Interestingly, research indicates that MSCs show promise as a treatment for various illnesses. Clinical and pre-clinical research is being done on the impact of MSCs on POF. According to the preliminary study findings, systemic administration of MSCs in clinical settings has significant therapeutic potential and will offer hope to POF patients. The clinical use of MSCs in POF will be available soon as a result of several successful clinical trials that have been accomplished [65]. hUC-MSCs can move into inflamed or injured tissue and develop into three separate germ layers, aiding in tissue healing [25]. Through various methods, MSCs obtained from multiple sources exhibit the same therapeutic effects in the therapy of POF. Even in elderly POF patients, MSCs offer to promise clinical transformation and practical potential for restoring reproductive function in POF patients. The molecular mechanism of POF is currently poorly understood, and this is a significant research problem for thoroughly assessing the efficacy and safety of transplantation of MSC, particularly the longer-term effect on parents and offspring [31, 93]. Based on previous investigations and core mechanistic studies, clinical study studies could significantly improve the treatment of ovarian dysfunction. There are more than 300 clinical trials for MSC therapy. Multipotent mesenchymal stromal cells (MSCs) are being investigated for nearly all therapeutic applications imaginable, according to more than 1050 clinical studies listed at FDA.gov. Numerous companies have commercialized or are currently commercializing MSC-based treatments. However, most MSC therapies have fallen short of meeting their primary efficacy end goals in clinical stages.
Concluding Remarks
Many couples experience grief and despair due to premature ovarian failure that contributes to infertility. Due to the complexity of this illness, no single therapy has been suggested; instead, combination treatments with fewer complications have been used. Though not particularly efficient, hormone therapy is typically used by POF patients to manage the symptoms. Over the past ten years, many stem cell types with therapeutic promise have been employed to treat POF. Most studies demonstrated the effectiveness of stem cells in treating POF, with evidence showing that these cells may develop into ovarian follicles and regain ovarian function. BMDSC received the most excellent attention globally for treating various diseases, including POF. However, severe drawbacks in clinical trials, including discomfort associated with their acquisition, aging-related declines in transplant efficacy, and the potential for immunological rejection alternatives to BMSCs, are suggested, such as Mesenchymal stem cells [85]. According to the preliminary findings of pre-clinical, clinical, and laboratory-based investigations, MSC is promising for treating POF. Numerous studies suggest that human mesenchymal stem cells offer promising clinical transformation and application potential in POF patients to fundamentally restore ovarian function because they are easily accessible and differentiate into most tissues. MSCs obtained from multiple sources and methods exhibit the same therapeutic effects in restoring reproductive function in POF patients [93, 94]. The therapeutic action of MSCs is generally regulated by a complex web of biological processes rather than a single component. Following MSC migration to the damaged ovary, paracrine effects control ovarian cell proliferation, induction of apoptosis and autophagy, immunization, fibrosis, and oxidative stress, low immunogenicity, variety of origins, and affordability takes place.
The mesenchymal stem cells that are extracted from the human umbilical cord (hUC-MSCs) comprise umbilical cord tissue-derived stem cells, and they retain not only mesenchymal stem cells' essential characteristics but also have a strong capacity for proliferation and differentiation. UCMSCs are exceeding becoming an acceptable substitute for BMSCs in therapeutic settings due to their several benefits, such as a Non-invasive collecting approach for allogeneic or autologous usage and their safe mode of harvesting that reduces the chances of an ethical conflict. The reason is hUC-MSCs pose no danger or impairment to the mother or the child, and 90% percent of human cords can quickly be separated to provide their considerable amounts. They are a dependable source of MSC for therapeutic use, given that they can be frozen and thawed, clonally propagated, altered to generate foreign proteins, and cultured in culture. Their Multipotent characteristics, with a Low chance of teratoma (as they are not tumorigenic and reside in the mesenchymal tissues) and a lower immunological competency with high immunosuppressive capacity than BMSCs, seems to favors them great potential as a prospective therapy option for POF.
By enhancing hUC-MSCs' capacity to survive, and an effective delivery method to the ovary, new techniques are being developed to boost their effectiveness and render them more promising for use in clinical settings. Overall, the recent findings demonstrated in pre-clinical study by Zhang that on UCMSC transplantation, the indicators underlying ovarian function had been restored, depicting an improvement in the ovarian function of rats [95]. This study might offer a base for the clinical use of UCMSC to treat POF. However, a thorough signaling pathway investigation of the molecular mechanisms of damage and repair when UCMSCs are administered in POF-modeled rats is required. The clinical use of UCMSCs in POF needs further studies in a clinical setting to elucidate their mechanism of therapeutic action, and using UCMSCs without proper supervision could have negative repercussions. A critical scientific challenge remains in fully and thoroughly assessing the efficacy and safety of UCMSC transplantation, particularly the long-term effects on mother and child.
Challenges and Future Prospects
As apparent as it may seem that UCMSC-based therapy seems to be gaining momentum, and we expect many new pre-clinical and clinical investigations will likely be conducted when additional stem cell characteristics are discovered, it still faces some challenges. Considering the clinical application of UCMSCs has a prerequisite of safety and efficacy for human cell-based therapy, several concerns still need to be addressed as current studies lack in-depth studies and need more future work. Comprehensive experimental and mechanistic studies are to be carried out in pre-clinical and clinical settings to address many technical issues. These issues include the number of cells to be transplanted, the window of time chosen for transplantation, the length of the therapeutic effect, the rate of injection, the frequency of transplantation, and the administration technique. However, all the participants in recent clinical studies received intra-ovarian injections of UCMSCs and displayed zero severe side effects/complications related to the treatment. In the studies, the intravenous injection might be a method of choice with far less invasiveness and a relatively short recovery time. Future randomized clinical studies should include an appropriate control group for a realistic assessment of the technique's effectiveness in a chosen group of patients. Since few in-depth investigations are available, deciphering POF's molecular mechanism remains a significant research challenge. Using adequate mechanistic investigations, we can then accurately assess the effectiveness and safety of UCMSC transplantation, particularly its long-term effects on parents and children.
Although UCMSCs only carry out immunoregulatory functions in the target localized tissue and do not lead to systemic immune function suppression, the ability of MSC-based therapies to impair immune function and promote tumor growth is another risk concern. Nevertheless, the lack of adverse effects and the safety of MSCs-related therapies is a central priority of fundamental research and clinical trials. Whether large dosages of UCMSCs produce malignant transformation must be determined to rule out tumor initiation and progression caused by these cells.
While there have been no cases of MSC-related malignancies developing in human patients, it is still possible for tumors to occur because of their propensity to encourage metastatic growth. Areas of tissue damage and inflammation draw MSCs, and as part of their regular healing activity, MSCs can home into the cancerous microenvironment. Despite their paracrine functions and the production of numerous trophic factors, MSCs, which have immunosuppressive qualities in the tumor microenvironment, can be regulated by tumor cells and, in turn, control the development, proliferation, and metastasis of tumor cells. However, studies show that Wharton's jelly stem cell (hWJSC) lysates were proven to reduce tumor development when MSCs were used as a therapy, displaying tumor-suppressive effects via enhancing pro-apoptotic Bax and lowering/downregulating anti-apoptotic Bcl-2 and SURVIVIN genes on breast adenocarcinoma, osteosarcoma, and ovarian carcinoma cells [96].
The absence of standard systematic protocols for MSC isolation, culture, identification, preparation, and administration methods leads to inconsistent outcomes in therapeutic settings. The International Society put out a set of core standards for using human MSCs for Cellular Therapy. Even though these criteria are not entirely implemented in clinical trials, they serve as a crucial starting point for creating more specific clinical application standards. Developing standards for MSC preparation in clinical practice is necessary since the underlying therapeutic processes vary depending on the cell source, the particular illness, and the intended usage. Researchers suggest efficient resource management promotes mechanistic research on MSCs and direct clinical trials to produce high effectiveness in the future [97]. The potential of Induced pluripotent stem cells (iPSCs)-based approach to overcome the primary constraints of current, donor-derived MSC production processes seems promising, as it makes it possible to create an almost infinite number of MSCs from a single blood donation [98].
The encouraging outcomes of several productive laboratory-based studies and the pre-clinical or clinical results of MSCs-mediated treatment have prompted researchers to examine MSCs-based therapeutic strategies thoroughly. After standardizing the preparation and verifying the safety of application in clinical investigations, MSCs-derived treatments will eventually be used to address POF or numerous difficult clinical conditions.
Data Availability
All the data and material of this manuscript will be accessible to the readers.
References
Persani, L., Rossetti, R., & Cacciatore, C. (2010). Genes involved in human premature ovarian failure. Journal of molecular endocrinology, 45(5), 257–279. https://doi.org/10.1677/JME-10-0070
Drummond, A. E. (2006). The role of steroids in follicular growth. Reproductive biology and endocrinology : RB&E, 4,. https://doi.org/10.1186/1477-7827-4-16
Sun, Y. C., Sun, X. F., Dyce, P. W., Shen, W., & Chen, H. (2017). The role of germ cell loss during primordial follicle assembly: A review of current advances. International Journal of Biological Sciences, 13(4), 449–457. https://doi.org/10.7150/IJBS.18836
Pangas, S. A., & Rajkovic, A. (2015). Follicular Development: Mouse, Sheep, and Human Models. Knobil and Neill’s Physiology of Reproduction: Two-Volume Set, 1, 947–995. https://doi.org/10.1016/B978-0-12-397175-3.00021-1
Zhao, Y. X., Chen, S. R., Su, P. P., Huang, F. H., Shi, Y. C., Shi, Q. Y., & Lin, S. (2019). Using Mesenchymal Stem Cells to Treat Female Infertility: An Update on Female Reproductive Diseases. Stem Cells International, 2019,. https://doi.org/10.1155/2019/9071720
Sheikhansari, G., Aghebati-Maleki, L., Nouri, M., Jadidi-Niaragh, F., & Yousefi, M. (2018). Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomedicine and Pharmacotherapy, 102(December 2017), 254–262. https://doi.org/10.1016/j.biopha.2018.03.056
Shamilova, N. N., Marchenko, L. A., Dolgushina, N. V., Zaletaev, D. V., & Sukhikh, G. T. (2013). The role of genetic and autoimmune factors in premature ovarian failure. Journal of Assisted Reproduction and Genetics, 30(5), 617–622. https://doi.org/10.1007/s10815-013-9974-4
Nelson, L. M. (2009). NIH Public Access - Primary Ovarian Insufficiency. New England Journal of Medicine, 360(11), 606–614. https://doi.org/10.1056/NEJMcp0808697.Primary
Conte, B., & Del Mastro, L. (2017). Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in breast cancer patients. Minerva ginecologica, 69(4), 350–356. https://doi.org/10.23736/S0026-4784.17.04067-9
Podfigurna-Stopa, A., Czyzyk, A., Grymowicz, M., Smolarczyk, R., Katulski, K., Czajkowski, K., & Meczekalski, B. (2016). Premature ovarian insufficiency: The context of long-term effects. Journal of Endocrinological Investigation, 39(9), 983. https://doi.org/10.1007/S40618-016-0467-Z
Kuang, H., Han, D., Xie, J., Yan, Y., Li, J., & Ge, P. (2013). Profiling of differentially expressed microRNAs in premature ovarian failure in an animal model. Gynecol Endocrinol, 30(1), 57–61. https://doi.org/10.3109/09513590.2013.850659
Şükür, Y. E., Kıvançlı, İB., & Özmen, B. (2014). Ovarian aging and premature ovarian failure. Journal of the Turkish German Gynecological Association, 15(3), 190. https://doi.org/10.5152/JTGGA.2014.0022
Goswami, D., & Conway, G. S. (2005). Premature ovarian failure. Human Reproduction Update, 11(4), 391–410. https://doi.org/10.1093/HUMUPD/DMI012
Kalantaridou, S. N., Davis, S. R., & Nelson, L. M. (1998). PREMATURE OVARIAN FAILURE. Endocrinology and Metabolism Clinics of North America, 27(4), 989–1006. https://doi.org/10.1016/S0889-8529(05)70051-7
Cramer, D. W., Xu, H., & Harlow, B. L. (1995). Family history as a predictor of early menopause. Fertility and Sterility, 64(4), 740–745. https://doi.org/10.1016/S0015-0282(16)57849-2
Goswami, D., & Conway, G. S. (2007). Premature Ovarian Failure. Hormone Research in Paediatrics, 68(4), 196–202. https://doi.org/10.1159/000102537
Van Kasteren, Y. M., & Schoemaker, J. (1999). Premature ovarian failure: A systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Human Reproduction Update, 5(5), 483–492. https://doi.org/10.1093/HUMUPD/5.5.483
Qin, Y., Jiao, X., Simpson, J. L., & Chen, Z. J. (2015). Genetics of primary ovarian insufficiency: New developments and opportunities. Human Reproduction Update, 21(6), 787–808. https://doi.org/10.1093/HUMUPD/DMV036
Hewlett, M., & Mahalingaiah, S. (2015). Update on Primary Ovarian Insufficiency. Current opinion in endocrinology, diabetes, and obesity, 22(6), 483. https://doi.org/10.1097/MED.0000000000000206
Wing, T., Yeung, Y., Hang, R., Li, W., Chi, V., Lee, Y., … Ng, Y. (2013). A Randomized Double-Blinded Placebo-Controlled Trial on the Effect of Dehydroepiandrosterone for 16 Weeks on Ovarian Response Markers in Women with Primary Ovarian Insufficiency. jcem.endojournals.org J Clin Endocrinol Metab, 98(1), 380–388. https://doi.org/10.1210/jc.2012-3071
Flöter, A., Nathorst-Böös, J., Carlström, K., & Von Schoultz, B. (2009). Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. 5(4), 357–365. 10.1080/CMT.5.4.357.365
Boothby, L. A., Doering, P. L., & Kipersztok, S. (2004). Bioidentical hormone therapy: a review. Menopause (New York, N. Y.), 11(3), 356–367. https://doi.org/10.1097/01.GME.0000094356.92081.EF
Paulson, R. J., Hatch, I. E., Lobo, R. A., & Sauer, M. V. (1997). Cumulative conception and live birth rates after oocyte donation: Implications regarding endometrial receptivity. Human reproduction (Oxford, England), 12(4), 835–839. https://doi.org/10.1093/HUMREP/12.4.835
Morgan, S., Anderson, R. A., Gourley, C., Wallace, W. H., & Spears, N. (2012). How do chemotherapeutic agents damage the ovary? Human Reproduction Update, 18(5), 525–535. https://doi.org/10.1093/HUMUPD/DMS022
Fu, Y. X., Ji, J., Shan, F., Li, J., & Hu, R. (2021). Human mesenchymal stem cell treatment of premature ovarian failure: New challenges and opportunities. Stem Cell Research and Therapy, 12(1), 1–13. https://doi.org/10.1186/s13287-021-02212-0
Wu, J. X., Xia, T., She, L. P., Lin, S., & Luo, X. M. (2022). Stem Cell Therapies for Human Infertility: Advantages and Challenges. Cell Transplantation, 31,. https://doi.org/10.1177/09636897221083252
Sun, L., Li, D., Song, K., Wei, J., Yao, S., Li, Z., … Li, L. (2017). Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Scientific Reports 2017 7:1, 7(1), 1–13. https://doi.org/10.1038/s41598-017-02786-x
Atala, A. (2007). Engineering tissues, organs and cells. Journal of tissue engineering and regenerative medicine, 1(2), 83–96. https://doi.org/10.1002/TERM.18
Qamar, A. Y., Hussain, T., Rafique, M. K., Bang, S., Tanga, B. M., Seong, G., … Cho, J. (2021). The Role of Stem Cells and Their Derived Extracellular Vesicles in Restoring Female and Male Fertility. Cells, 10(9). https://doi.org/10.3390/CELLS10092460
Wang, J., Liu, C., Fujino, M., Tong, G., Zhang, Q., Li, X.-K., & Yan, H. (2019). Stem Cells as a Resource for Treatment of Infertility-related Diseases. Current Molecular Medicine, 19(8), 519. https://doi.org/10.2174/1566524019666190709172636
Wang, M. Y., Wang, Y. X., Li-Ling, J., & Xie, H. Q. (2022). Adu1. Wang MY, Wang YX, Li-Ling J, Xie HQ. Adult Stem Cell Therapy for Premature Ovarian Failure: From Bench to Bedside. Tissue Eng - Part B Rev. 2022;28(1):63–78. doi:https://doi.org/10.1089/ten.teb.2020.0205lt Stem Cell Therapy for Premature Ovarian Failure: From Bench. Tissue Engineering - Part B: Reviews, 28(1), 63–78. https://doi.org/10.1089/ten.teb.2020.0205
Lee, H. J., Selesniemi, K., Niikura, Y., Niikura, T., Klein, R., Dombkowski, D. M., & Tilly, J. L. (2007). Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. Journal of clinical oncology : Official journal of the American Society of Clinical Oncology, 25(22), 3198–3204. https://doi.org/10.1200/JCO.2006.10.3028
Kawamura, K., Kawamura, N., & Hsueh, A. J. W. (2016). Activation of dormant follicles: A new treatment for premature ovarian failure? Current opinion in obstetrics & gynecology, 28(3), 217–222. https://doi.org/10.1097/GCO.0000000000000268
Gupta, S., Lodha, P., Karthick, M. S., & Tandulwadkar, S. (2018). Role of Autologous Bone Marrow-Derived Stem Cell Therapy for Follicular Recruitment in Premature Ovarian Insufficiency: Review of Literature and a Case Report of World’s First Baby with Ovarian Autologous Stem Cell Therapy in a Perimenopausal Woman of Age. Journal of human reproductive sciences, 11(2), 125–130. https://doi.org/10.4103/JHRS.JHRS_57_18
Squillaro, T., Peluso, G., & Galderisi, U. (2016). Clinical Trials With Mesenchymal Stem Cells: An Update. Cell transplantation, 25(5), 829–848. https://doi.org/10.3727/096368915X689622
Weiss, M. L., & Troyer, D. L. (2006). Stem cells in the umbilical cord. Stem cell reviews, 2(2), 155–162. https://doi.org/10.1007/S12015-006-0022-Y
Shareghi-oskoue, O., Aghebati-Maleki, L., & Yousefi, M. (2021). Transplantation of human umbilical cord mesenchymal stem cells to treat premature ovarian failure. Stem Cell Research & Therapy, 12(1), 1–13. https://doi.org/10.1186/S13287-021-02529-W
Ullah, I., Subbarao, R. B., & Rho, G. J. (2015). Human mesenchymal stem cells-current trends and future prospective. Bioscience Reports, 35(2). https://portlandpress.com/bioscirep/article/35/2/e00191/56141/Human-mesenchymal-stem-cells-current-trends-and
Esfandyari, S., Chugh, R. M., Park, H. S., Hobeika, E., Ulin, M., & Al-Hendy, A. (2020). Mesenchymal stem cells as a bio organ for treatment of female infertility. Cells, 9(10), 2253. https://www.mdpi.com/2073-4409/9/10/2253
Naji, A., Rouas-Freiss, N., Durrbach, A., Carosella, E. D., Sensébé, L., & Deschaseaux, F. (2013). Concise review: Combining human leukocyte antigen G and mesenchymal stem cells for immunosuppressant biotherapy. Stem cells (Dayton, Ohio), 31(11), 2296–2303. https://doi.org/10.1002/STEM.1494
Galipeau, J., & Sensébé, L. (2018). Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell, 22(6), 824–833. https://doi.org/10.1016/J.STEM.2018.05.004
Trounson, A., & McDonald, C. (2015). Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell, 17(1), 11–22. https://doi.org/10.1016/J.STEM.2015.06.007
Zhang, S., Zhu, D., Mei, X., Li, Z., Li, J., Xie, M., … & Cheng, K. (2021). Advances in biomaterials and regenerative medicine for primary ovarian insufficiency therapy. Bioactive Materials, 6(7), 1957–1972. https://doi.org/10.1016/J.BIOACTMAT.2020.12.008
Asgari, H. R., Akbari, M., Abbasi, M., Ai, J., Korouji, M., Aliakbari, F., … Joghataei, M. T. (2015). Human Wharton’s jelly-derived mesenchymal stem cells express oocyte developmental genes during co-culture with placental cells. Iranian Journal of Basic Medical Sciences, 18(1), 22. Retrieved from /pmc/articles/PMC4366739/
Ding, D. C., Chang, Y. H., Shyu, W. C., & Lin, S. Z. (2015). Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplantation, 24(3), 339–347. https://doi.org/10.3727/096368915X686841
Zhang, H., Tao, Y., Liu, H., Ren, S., Zhang, B., Immunology, H. C.-M., & 2017, undefined. (n.d.). Immunomodulatory function of whole human umbilical cord derived mesenchymal stem cells. Elsevier. Retrieved from https://www.sciencedirect.com/science/article/pii/S0161589017300743, https://doi.org/10.1016/j.molimm.2017.03.003. Accessed 5 Aug 2022
Kobolak, J., Dinnyes, A., Memic, A., Khademhosseini, A., & Mobasheri, A. (2016). Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro. Elsevier, 99, 62–68. https://doi.org/10.1016/j.ymeth.2015.09.016
Thaweesapphithak, S., Tantrawatpan, C., Kheolamai, P., Tantikanlayaporn, D., Roytrakul, S., & Manochantr, S. (2019). Human serum enhances the proliferative capacity and immunomodulatory property of MSCs derived from human placenta and umbilical cord. Stem Cell Research & Therapy, 10(1), 1–18. https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1175-3
Varaa, N., Azandeh, S., Khodabandeh, Z., & Gharravi, A. M. (2019). Wharton’s Jelly Mesenchymal Stem Cell: Various Protocols for Isolation and Differentiation of Hepatocyte-Like Cells; Narrative Review. Iranian Journal of Medical Sciences, 44(6), 437. https://doi.org/10.30476/IJMS.2019.44952
Kita, K., Gauglitz, G. G., Phan, T. T., Herndon, D. N., & Jeschke, M. G. (2010). Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem cells and development, 19(4), 491–501. https://doi.org/10.1089/SCD.2009.0192
Han, Y. F., Tao, R., Sun, T. J., Chai, J. K., Xu, G., & Liu, J. (2013). Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology, 65(5), 819. https://doi.org/10.1007/S10616-012-9528-0
Li, D. R., & Cai, J. H. (2012). Methods of isolation, expansion, differentiating induction and preservation of human umbilical cord mesenchymal stem cells. Chinese Medical Journal, 125(24), 4504–4510. https://doi.org/10.3760/CMA.J.ISSN.0366-6999.2012.24.032
Nagamura-Inoue, T., & He, H. (2014). Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World journal of stem cells, 6(2), 195–202. https://doi.org/10.4252/wjsc.v6.i2.195
Troyer, D. L., & Weiss, M. L. (2008). Concise Review: Wharton’s Jelly-Derived Cells Are a Primitive Stromal Cell Population. Stem Cells (Dayton, Ohio), 26(3), 591. https://doi.org/10.1634/STEMCELLS.2007-0439
Sdrimas, K., & Kourembanas, S. (2014). MSC Microvesicles for the Treatment of Lung Disease: A New Paradigm for Cell-Free Therapy. Antioxidants & Redox Signaling, 21(13), 1905. https://doi.org/10.1089/ARS.2013.5784
Wang, Z., Wang, Y., Yang, T., Li, J., & Yang, X. (2017). Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Research & Therapy, 8(1), 1–14. https://doi.org/10.1186/S13287-016-0458-1
Phinney, D. G., & Pittenger, M. F. (2017). Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. STEM CELLS, 35(4), 851–858. https://doi.org/10.1002/STEM.2575
Hu, L., Hu, J., Zhao, J., Liu, J., Ouyang, W., Yang, C., … & Chen, L. (2013). Side-by-side comparison of the biological characteristics of human umbilical cord and adipose tissue-derived mesenchymal stem cells. BioMed research international, 2013,. https://doi.org/10.1155/2013/438243
Li, J., Mao, Q., He, J., She, H., Zhang, Z., & Yin, C. (2017). Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Research & Therapy, 8(1), 1–11. https://stemcellres.biomedcentral.com/articles/10.1186/s13287-017-0514-5
He, Y., Chen, D., Yang, L., Hou, Q., Ma, H., & Xu, X. (2018). The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Research & Therapy, 9(1), 1–7. https://link.springer.com/article/10.1186/s13287-018-1008-9
Jacobs, S. A., Roobrouck, V. D., Verfaillie, C. M., & Van Gool, S. W. (2013). Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunology and Cell Biology, 91(1), 32. https://doi.org/10.1038/ICB.2012.64
Klyushnenkova, E., Mosca, J. D., Zernetkina, V., Majumdar, M. K., Beggs, K. J., Simonetti, D. W., … & Mcintosh, K. R. (2005). T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. Springer, 12(1), 47–57. https://doi.org/10.1007/s11373-004-8183-7
Van Gool, S. W., Vandenberghe, P., de Boer, M., & Ceuppens, J. L. (1996). CD80, CD86 and CD40 Provide Accessory Signals in a Multiple-Step T-Cell Activation Model. Immunological Reviews, 153(1), 47–83. https://doi.org/10.1111/J.1600-065X.1996.TB00920.X
Le Blanc, K., Tammik, L., Sundberg, B., Haynesworth, S. E., & Ringdén, O. (2003). Mesenchymal Stem Cells Inhibit and Stimulate Mixed Lymphocyte Cultures and Mitogenic Responses Independently of the Major Histocompatibility Complex. Scandinavian Journal of Immunology, 57(1), 11–20. https://doi.org/10.1046/J.1365-3083.2003.01176.X
Fu, Z. J., Li, X. D., Wei, D. W., & Ding, X. L. (2021). Clinical application potential of umbilical cord mesenchymal stem cells in chemotherapeutic ovarian failure. Reproductive and Developmental Medicine, 5(1), 55. https://doi.org/10.4103/2096-2924.313685
Nicola, M. D., Carlo-Stella, C., Magni, M., Milanesi, M., Longoni, P. D., Matteucci, P., … & Gianni, A. M. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood, 99(10), 3838–3843. https://doi.org/10.1182/BLOOD.V99.10.3838
Lu, X., Cui, J., Cui, L., Luo, Q., Cao, Q., Yuan, W., & Zhang, H. (2019). The effects of human umbilical cord-derived mesenchymal stem cell transplantation on endometrial receptivity are associated with Th1/Th2 balance change and uNK cell expression of uterine in autoimmune premature ovarian failure mice. Stem Cell Research and Therapy, 10(1), 1–11. https://doi.org/10.1186/s13287-019-1313-y
Yin, N., Zhao, W., Luo, Q., Yuan, W., Luan, X., & Zhang, H. (2018). Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by Treg cells and associated cytokines. Reproductive Sciences, 25(7), 1073–1082. https://doi.org/10.1177/1933719117732156
Wang, Z., Wei, Q., Wang, H., Han, L., Dai, H., Qian, X., & Qi, N. (2020). Mesenchymal Stem Cell Therapy Using Human Umbilical Cord in a Rat Model of Autoimmune-Induced Premature Ovarian Failure. Stem Cells International, 2020,. https://doi.org/10.1155/2020/3249495
Wang, M., Yuan, Q., & Xie, L. (2018). Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells International, 2018,. https://doi.org/10.1155/2018/3057624
Cui, L., Bao, H., Liu, Z., Man, X., Liu, H., Hou, Y., … & Zhang, H. (2020). HUMSCs regulate the differentiation of ovarian stromal cells via TGF-β1/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats. Stem Cell Research and Therapy, 11(1), 1–12. https://doi.org/10.1186/S13287-020-01904-3/FIGURES/6
Polonio, A. M., García-Velasco, J. A., & Herraiz, S. (2021). Stem Cell Paracrine Signaling for Treatment of Premature Ovarian Insufficiency. Frontiers in endocrinology, 11,. https://doi.org/10.3389/FENDO.2020.626322
Hung, T. H., Hsieh, C. C., Hsu, J. J., Lo, L. M., Chiu, T. H., & Hsieh, T. T. (2007). Risk factors for placental abruption in an Asian population. Reproductive Sciences, 14(1), 59–65. https://doi.org/10.1177/1933719106298363
Lin, P., Correa, D., Lin, Y., & Caplan, A. I. (2011). Polybrene Inhibits Human Mesenchymal Stem Cell Proliferation during Lentiviral Transduction. PLoS ONE, 6(8), e23891. https://doi.org/10.1371/JOURNAL.PONE.0023891
Park, D. H., Eve, D. J., Chung, Y. G., & Sanberg, P. R. (2010). Regenerative Medicine for Neurological Disorders. The Scientific World Journal, 10, 470. https://doi.org/10.1100/TSW.2010.39
Kilic, S., Pinarli, F., Ozogul, C., Tasdemir, N., NazSarac, G., & Delibasi, T. (2014). Protection from cyclophosphamide-induced ovarian damage with bone marrow-derived mesenchymal stem cells during puberty. Gynecol Endocrinol, 30(2), 135–140. https://doi.org/10.3109/09513590.2013.860127
Yan, M., Sun, M., Zhou, Y., Wang, W., He, Z., Tang, D., … & Li, H. (2013). Conversion of Human Umbilical Cord Mesenchymal Stem Cells in Wharton’s Jelly to Dopamine Neurons Mediated by the Lmx1a and Neurturin In Vitro: Potential Therapeutic Application for Parkinson’s Disease in a Rhesus Monkey Model. PLoS ONE, 8(5), e64000. https://doi.org/10.1371/JOURNAL.PONE.0064000
Souidi, N., Stolk, M., & Seifert, M. (2013). Ischemia-reperfusion injury: Beneficial effects of mesenchymal stromal cells. Current Opinion in Organ Transplantation, 18(1), 34–43. https://doi.org/10.1097/MOT.0B013E32835C2A05
Yan, Y., Xu, W., Qian, H., Si, Y., Zhu, W., Cao, H., … & Mao, F. (2009). Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver International, 29(3), 356–365. https://doi.org/10.1111/J.1478-3231.2008.01855.X
Weiss, M. L., Anderson, C., Medicetty, S., Seshareddy, K. B., Weiss, R. J., VanderWerff, I., … & McIntosh, K. R. (2008). Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells, 26(11), 2865–2874. https://doi.org/10.1634/STEMCELLS.2007-1028
Yang, Y., Lei, L., Wang, S., Sheng, X., Yan, G., Xu, L., … Sun, H. (2019). Transplantation of umbilical cord-derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. In vitro cellular & developmental biology. Animal, 55(4), 302–311. https://doi.org/10.1007/S11626-019-00337-4
Fu, X., He, Y., Xie, C., & Liu, W. (2008). Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy, 10(4), 353–363. https://doi.org/10.1080/14653240802035926
Lu, X., Bao, H., Cui, L., Zhu, W., Zhang, L., Xu, Z., … & Zhang, H. (2020). hUMSC transplantation restores ovarian function in POI rats by inhibiting autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway. Stem Cell Research and Therapy, 11(1), 1–13. https://doi.org/10.1186/S13287-020-01784-7/FIGURES/7
Zhang, L., Sun, Y., Zhang, X.-X., Liu, Y.-B., Sun, H.-Y., Wu, C.-T., … Wang, L.-S. (2022). Comparison of CD146 +/− mesenchymal stem cells in improving premature ovarian failure. Stem Cell Research & Therapy, 13(1). https://doi.org/10.1186/s13287-022-02916-x
Zhang, X., Zhang, L., Li, Y., Yin, Z., Feng, Y., & Ji, Y. (2021). Human umbilical cord mesenchymal stem cells (hUCMSCs) promotes the recovery of ovarian function in a rat model of premature ovarian failure (POF). Gynecological Endocrinology, 37(4), 353–357. https://doi.org/10.1080/09513590.2021.1878133
Yan, L., Wu, Y., Li, L., Wu, J., Zhao, F., Gao, Z., … Wang, H. (2020). Clinical analysis of human umbilical cord mesenchymal stem cell allotransplantation in patients with premature ovarian insufficiency. Cell Proliferation, 53(12). https://doi.org/10.1111/CPR.12938
Edessy, M., Hosni, H. N., Shady, Y., Waf, Y., Bakr, S., & Kamel, M. (2016). Autologous stem cells therapy, The first baby of idiopathic premature ovarian failure. Acta Medica International, 3(1), 19. https://doi.org/10.5530/AMI.2016.1.7
Mehling, B. M., Manvelyan, M., & Wu, D.-C. (2016). Autologous stem cell transplantation in patients with idiopathic premature ovarian failure. Journal of Tissue Science and Engineering, 07(03), 3. https://doi.org/10.4172/2157-7552.C1.030
Herraiz, S., Buigues, A., Díaz-García, C., Romeu, M., Martínez, S., Gómez-Seguí, I., … & Pellicer, A. (2018). Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertility and sterility, 109(5), 908-918.e2. https://doi.org/10.1016/J.FERTNSTERT.2018.01.004
Herraiz, S., Romeu, M., Buigues, A., Martínez, S., Díaz-García, C., Gómez-Seguí, I., … & Pellicer, A. (2018). Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertility and sterility, 110(3), 496-505.e1. https://doi.org/10.1016/J.FERTNSTERT.2018.04.025
Liu, J., Zhang, H., Zhang, Y., Li, N., Wen, Y., Cao, F., … Xue, X. (2014). Homing and Restorative Effects of Bone Marrow-Derived Mesenchymal Stem Cells on Cisplatin Injured Ovaries in Rats. Molecules and Cells, 37(12), 865. https://doi.org/10.14348/MOLCELLS.2014.0145
Gnecchi, M., Zhang, Z., Ni, A., & Dzau, V. J. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circulation research, 103(11), 1204–1219. https://doi.org/10.1161/CIRCRESAHA.108.176826
Wang, J., Liu, W., Yu, D., Yang, Z., Li, S., & Sun, X. (2021). Research Progress on the Treatment of Premature Ovarian Failure Using Mesenchymal Stem Cells: A Literature Review. Frontiers in Cell and Developmental Biology, 9(December), 1–13. https://doi.org/10.3389/fcell.2021.749822
Li, Z., Zhang, M., Tian, Y., Li, Q., & Huang, X. (2021). Mesenchymal Stem Cells in Premature Ovarian Insufficiency: Mechanisms and Prospects. Frontiers in Cell and Developmental Biology, 9(August), 1–13. https://doi.org/10.3389/fcell.2021.718192
Zhang, M., Xie, T., Dai, W., Zhao, B., Zheng, Y., Hu, J., … & Wang, L. (2022). Umbilical Cord Mesenchymal Stem Cells Ameliorate Premature Ovarian Insufficiency in Rats. Evidence-Based Complementary and Alternative Medicine, 2022, 1–12. https://doi.org/10.1155/2022/9228456
Gauthaman, K., Yee, F. C., Cheyyatraivendran, S., Biswas, A., Choolani, M., & Bongso, A. (2012). Human umbilical cord Wharton’s jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. Journal of cellular biochemistry, 113(6), 2027–2039. https://doi.org/10.1002/JCB.24073
Sipp, D., Robey, P. G., & Turner, L. (2018). Clear up this stem-cell mess. Nature, 561(7724), 455–457. https://doi.org/10.1038/d41586-018-06756-9
Bloor, A. J. C., Patel, A., Griffin, J. E., Gilleece, M. H., Radia, R., Yeung, D. T., … Rasko, J. E. J. (2020). Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: a phase I, multicenter, open-label, dose-escalation study. Nature Medicine 2020 26:11, 26(11), 1720–1725. https://doi.org/10.1038/s41591-020-1050-x
Acknowledgements
The authors appreciated Mr. Muhammad Awais and Mr. Hamza Yaqoob for providing great help with the figures in this manuscript.
Funding
No funding received for this work.
Author information
Authors and Affiliations
Contributions
AU wrote the original draft and prepared the tables. NK revised the draft, and supervised the team. DLG reviewed and revised the draft. SS and UH assisted in the material collection of the original draft. AUK revised the original draft and assisted in writing conclusion, challenges and future prospects.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval and Consent to Publish
All the authors read and approve this manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umer, A., Khan, N., Greene, D.L. et al. The Therapeutic Potential of Human Umbilical Cord Derived Mesenchymal Stem Cells for the Treatment of Premature Ovarian Failure. Stem Cell Rev and Rep 19, 651–666 (2023). https://doi.org/10.1007/s12015-022-10493-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10493-y