Abstract
Improved understanding of the key mechanisms underlying cerebral ischemic injury is essential for the discovery of efficacious novel therapeutics for stroke. Through detailed analysis of plasma samples obtained from a large number of healthy volunteers (n = 90) and ischemic stroke patients (n = 81), the current study found significant elevations in the levels of TNF-α at baseline (within the first 48 h of stroke) and on days 7, 30, 90 after ischaemic stroke. It then assessed the impact of this inflammatory cytokine on an in vitro model of human blood–brain barrier (BBB) and revealed dramatic impairments in both barrier integrity and function, the main cause of early death after an ischemic stroke. Co-treatment of BBB models in similar experiments with outgrowth endothelial cell-derived conditioned media (OEC-CM) negated the deleterious effects of TNF-α on BBB. Effective suppression of anti-angiogenic factor endostatin, stress fiber formation, oxidative stress, and apoptosis along with concomitant improvements in extracellular matrix adhesive and tubulogenic properties of brain microvascular endothelial cells and OECs played an important role in OEC-CM-mediated benefits. Significant increases in pro-angiogenic endothelin-1 and monocyte chemoattractant protein-1 in OEC-CM compared to the secretomes of OEC and HBMEC, detected by proteome profiling assay, accentuate the beneficial effects of OEC-CM. In conclusion, this reverse translational study identifies TNF-α as an important mediator of post-ischemic cerebral barrier damage and proposes OEC-CM as a potential vasculoprotective therapeutic strategy by demonstrating its ability to regulate a wide range of mechanisms associated with BBB function. Clinical trial registration NCT02980354.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke (IS), stemming from an interference with blood supply to the brain, continues to be one of the leading causes of mortality and morbidity worldwide [1, 2]. Thrombolysis with intravenous recombinant tissue plasminogen activator (rtPA) and mechanical thrombectomy are the only approved treatment options for acute IS. However, due to short therapeutic windows associated with these treatments (up to 4.5 h and 24 h, respectively), each year < 1% of patients receive these therapies globally [3,4,5]. The complex pathophysiology of stroke involves many processes, including ATP depletion, excitotoxicity, inflammation, oxidative stress, necrosis, apoptosis and an increase in intracellular calcium levels [6, 7]. Hence, a better understanding of mechanisms involved in the pathogenesis and outcome of stroke is of paramount importance [8]. In this context, reverse translational studies, working from bedside to bench, may be of enormous help to uncover the main causative factors and help inform the design of future therapeutic strategies [9, 10]. Through detailed analysis of plasma samples of IS patients and healthy volunteers (HVs) recruited for The Dunhill Medical Trust EPC (DMT EPC) study, this study has identified inflammatory cytokine tumor necrosis factor-α (TNF-α) as an important factor for the progression and outcome of IS and showed significant elevations in its level during acute, subacute and chronic phases of the disease [11].
Again, considering that the majority of mortalities occur within the first week of an IS due to disruption of blood–brain barrier (BBB) and ensuing formation of cerebral edema, restoration of BBB function is now regarded as an important therapeutic priority [7, 12]. Endothelial progenitor cells (EPCs) and their functional subtype, called outgrowth endothelial cells (OECs), may make one such therapeutics. EPCs/OECs can differentiate into mature ECs and release a wide range of trophic factors to maintain BBB integrity in all (physio)pathological settings [13]. Indeed, recovery of BBB and neurological functions in stroke rats treated with OECs 24–48 h after middle cerebral artery occlusion (MCAo) confirmed the ability of these cells to engraft into brain capillaries and serve as therapeutics [14, 15]. However, replicative senescence of OECs during large scale ex vivo expansion and elevated risk of emboli formation and immune reaction associated with cell-based therapies have thus far hampered the widespread use of these particular cells in clinical practice [16].
As post-ischemic neurovascular reparative function of OECs is widely attributed to a wide range of factors, e.g. angiogenin, interleukin-8, platelet-derived growth factor-BB (PDGF-BB) and vascular endothelial cell growth factor (VEGF) that they secrete, OEC-derived conditioned medium (OEC-CM) has attracted attention as an alternative therapeutic [17, 18]. In addition to addressing most side effects associated with cell-based therapies, an OEC-CM-based therapeutic approach also offers benefits concerning the ease of production, handling, storage, and cost [17, 19].
In light of the above, using a well-established in vitro model of human BBB, this reverse translational study aims to investigate whether OEC-CM can protect BBB from the deleterious effects of TNF-α and attempts to delineate mechanisms involved in its putative beneficial effects.
Materials and Methods
Study Design
DMT EPC study followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting. The study protocol was reviewed and approved by West Midlands—Coventry & Warwickshire Research Ethics Committee (16/WM/0304). Although details of the study have previously been described elsewhere, it is of note that individuals with ≥ 140/90 mmHg blood pressure, ≥ 7 mmol/L fasting glucose or 11.1 mmol/L 2 h post-prandial glucose level and with ≥ 5 mmol/L total cholesterol level were successively considered as hypertensive, diabetic and hyperlipidaemic [11].
In brief, the DMT EPC investigates the quantity and functionality of circulating EPCs as well as the biochemical profile of HVs and IS patients at four different timepoints following ischemic injury: days 0 (within 48 h of IS symptom onset), 7, 30, and 90, covering acute, subacute, and chronic phases of IS (Supplementary Fig. 1). To this end, 30 mL blood samples were taken from each participant at each timepoint and were split for the abovementioned objectives. The first 6 mL were used to quantify the number of circulating EPCs by flow cytometry, and the plasma was simultaneously extracted to assess the biochemical profiles of participants, while the remaining blood was used to produce OECs.
Biochemical Assays
Specific ELISAs were used as per the manufacturers’ (all from R&D Systems unless otherwise stated) instructions to assess the plasma levels of TNF-α, granulocyte colony-stimulating factor (G-CSF), PDGF-BB, stromal derived factor-1 (SDF-1), total anti-oxidant capacity (Abcam), VEGF, endostatin, angiostatin (Abcam), thrombospondin-1, and thrombospondin-2.
Cell Culture
Human brain microvascular endothelial cells (HBMECs), pericytes, and astrocytes were purchased from TCS CellWorks Ltd. (Buckingham, UK) and cultured at 37 °C in a humidified atmosphere (75% N2, 20% O2, 5% CO2) with their respective media (Sciencell Research Laboratories, San Diego, USA). Mononuclear cells isolated from a 24-ml of human peripheral blood sample were cultured on fibronectin-coated plates to encourage the growth of OECs as previously reported [13]. The Endothelial Basal Medium-2 (EBM-2, Lonza), containing 20% FBS and all the supplements provided with the medium, was used to culture OECs. Once obtained, OECs were characterized, as cells possessing both endothelial and progenitor cell properties, using a series of morphological, immunophenotypic, and tubulogenic assessments as before [13].
Due to consistent elevations observed in HBMEC oxidative stress and BBB permeability with 10 ng/mL TNF-α for 6 h, this particular timepoint and concentration were used throughout the current study [20].
Acquisition of OEC-CM
OEC-CM was generated by culturing ~ 90% confluent OECs, isolated from the blood samples of three different healthy individuals, with EBM-2 containing 1% FBS but lacking all other supplements in hypoxic conditions (1.5% O2, 5% CO2, 93.5% N2) for 48 h [18]. This was followed by collection, centrifugation (10,000 rpm at 4 °C), filtration (via a 0.22 μm filter), and snap freezing of supernatant at -80 °C. EBM-2 containing 1% FBS and lacking all other supplements was used as a control medium.
Proteome Profiling of Cells
A proteome profiler human angiogenesis array (R&D Systems) was used to assess the angiogenic factors released from the cells as per the manufacturer’s instructions. To generate OEC and HBMEC secretomes, cells were cultured under normal conditions (75% N2, 20% O2, 5% CO2) for 48 h in EBM-2 containing solely 1% FBS. OEC-CM, generated as above, were also used in the studies.
Establishment of a Triple Cell Culture Model of BBB
For this, ~ 7.5 × 104 human astrocytes (HAs) were seeded on the basal side of polyester Transwell inserts (0.4 µm pore size, 12 mm diameter polyester membrane, High Wycombe, UK). On the following day, the inserts were inverted the right way and placed into a 12-well plate containing fresh medium. Once astrocytes reached ~ 90% confluence, HBMEC (~ 5 × 104 cells), alone or mixed with OECs (2:1 HBMEC:OEC ratio), were seeded onto the apical side of the inserts. When both cell layers were fully confluent, Transwell inserts were transferred to 12-well plates containing confluent human pericytes (HPs) to establish the triple cell culture model of human BBB [21].
Assessment of BBB Integrity and Function
The BBB integrity and function were assessed as before by measurements of transendothelial electrical resistance (TEER, World Precision Instruments, Hertfordshire, UK) and paracellular flux of the low molecular weight permeability marker, sodium fluorescein (NaF, 376 Da), respectively [21].
Measurement of NADPH Oxidase Capacity and Superoxide Anion Level
NADPH oxidase activity and the levels of superoxide anion were measured by lucigenin chemiluminescence and cytochrome-C reduction assays, respectively [22].
Wound Scratch Assay
OECs and HBMECs (1.5 × 104 cells/well) were seeded in 6-well plates with their specialized media until reaching 90% confluence. A scratch was then made by scraping the cell layer across each culture plate using the tip of a p1000 micropipette in one swift motion. The cells were washed with phosphate-buffered saline (PBS) to remove the debris. Pictures of wound closure were taken immediately after the scratch (0 h) and 24 h after this. Wound closure was quantified as the percentage of difference in scratch area between 0 and 24 h using ImageJ software (version 1.52 k, NIH, Maryland, USA).
Proliferation Assay
Cellular proliferation rates were determined using the 4-[3-(4-lodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1.3-benzene disulfonate (WST-1) kit (Roche, Mannheim, Germany). For this, ~ 5 × 103 cells, seeded and cultured in a 96-well plate for 48 h, were firstly exposed to the respective experimental conditions. 100 μL fresh medium replacement containing 10 μL of WST-1 was then added prior to a further 2 h incubation at 37 °C. The absorbance (450 nm) was subsequently read, using a FLUOstar Omega plate reader (BMG Labtech Ltd., UK).
Tube Formation Assay
Firstly, growth factor-reduced Matrigel (50 μL/well, BD Biosciences) was added to 96-well plates and allowed to polymerize at 37 °C. 1 × 104 cells were then seeded to each well and subjected to respective experimental conditions. The specific characteristics of the network of tubules, defined as the sum of number or length of segments, isolated elements and branches detected in the analyzed area, were then assessed using ImageJ software (version 1.52 k, NIH, Maryland, USA) [23].
Cell Adhesion Assay
Equal number of cells, grown in 6-well plates and exposed to experimental conditions, were seeded on fibronectin (Sigma) or collagen (Sigma) pre-coated 96-well plates. Following 1 h incubation, the plates were gently washed with PBS and examined under light microscope. The adherent cells were counted in at least four random fields per well.
Cell Viability and Caspase 3/7 Assay
Calcein AM (Calbiochem, Massachusetts, USA) and Apo-ONE homogeneous caspase-3/7 assay kit (Promega, Southampton, UK) were used to measure cell viability and caspase 3/7 activity, respectively as described before [24].
Immunocytochemistry
The organization of actin cytoskeleton was studied by staining of F-actin filaments with rhodamine phallodin as before [25]. Cells grown on coverslips were fixed in 4% paraformaldehyde/PBS for 20 min and permeabilized with 0.1% Triton X-100/PBS for 15 min before staining with 1 × rhodamine phalloidin for 60 min (Abcam, Cambridge, UK). The coverslips were then mounted on glass slides prior to visualization with fluorescence microscopy (Zeiss Axio Observer, Carl Zeiss Ltd., Cambridge, UK). The number of stress fibres was manually counted.
Detection of Apoptosis
Cells, subjected to different experimental conditions, were fixed in 4% paraformaldehyde/PBS for 20 min and stained for 5 min at room temperature with Hoechst 33,258 (10 μg/mL, Sigma). The coverslips were mounted on glass slides and analyzed by fluorescence microscopy (Zeiss Axio Observer, Carl Zeiss Ltd., Cambridge, UK).
Statistical Analyses
The statistical analyses were performed either with GraphPad Prism 8.0 statistical software package (GraphPad Software Inc., La Jolla, Ca, USA) or SPSS package 15.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Statistical tests were performed by t-test, Mann–Whitney, chi-square, or one-way analysis of variance followed by Tukey’s post hoc analysis, where appropriate. P < 0.05 was considered as significant.
Results
Patients with IS Display Significant Variations in Inflammatory and Angiogenic Modulator Generation
A total of 90 HVs and 81 patients were recruited for the study. Table 1 summarizes the demographical and clinical data collected for all participants. Compared to HVs, IS patients had substantially higher TNF-α levels which remained elevated at all timepoints covered in the study (Fig. 1A). In light of the etiological differences between cortical and lacunar strokes, i.e. large artery disease and endothelial dysfunction, respectively, this study specifically assessed the levels of plasma TNF-α in both subgroups and found similar readings (Supplementary Fig. 2).
The plasma level of major elements that have relevance to endothelial function in patients with IS and HVs. (A-D) The levels of pro-inflammatory mediator TNF-α and chemoattractant SDF-1 increased in patients at different phases of ischemic stroke while no differences were observed in the levels of G-CSF and total antioxidant capacity. (E, F) Gradual decreases observed in pro-angiogenic factors, VEGF and PDGF-BB reached significance at day 90 of ischemic stroke. (G-J) Ischemic stroke differentially regulated the levels of angiogenic suppressors in that the levels of endostatin constantly increased after ischemic injury while the levels of thrombospondin-1, and thrombospondin-2 decreased. No change was observed in angiostatin levels. * < 0.05, **P < 0.01, ***P < 0.001 versus HVs (t test or Mann Whitney U test). NS: not significant compared to HVs. G-CSF, granulocyte colony-stimulating factor; HVs, healthy volunteers; IS, ischemic stroke; PDGF-BB, platelet-derived growth factor; SDF-1, stromal cell-derived factor 1; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor
Similarly, the level of chemokine SDF-1, known to mediate trafficking and homing of EPCs to the site of injury, was also higher in patients which peaked on day 7, gradually decreased from then on and normalized on day 90 (Fig. 1B). In contrast, no changes in the plasma level of cytokine G-CSF, known to mobilize EPCs, were observed between patients and HVs at any timepoint (Fig. 1C).
Oxidative stress, emerging from an imbalance between endogenous pro-oxidants and anti-oxidants, is thought to profoundly affect the pathogenesis and outcome of IS [26]. Analyses of the plasma total anti-oxidant capacity, determined by the sum of food-derived and endogenous anti-oxidants including the enzymes, small molecules and proteins (e.g. catalase, ascorbate, and albumin), have shown insignificant decreases in IS patients compared to HVs (Fig. 1D).
Effective neovascularization of infarct area, mediated by release of pro-angiogenic factors in the injured tissue, is crucial for recovery following an IS [27]. Gradual decreases in plasma levels of major pro-angiogenic factors, VEGF and PDGF-BB, reaching significance on day 90 after stroke compared to HVs, may in part explain the differences in stroke patients’ outcome (Fig. 1E, F). Interestingly, plasma levels of anti-angiogenic factors thrombospondin-1 and thrombospondin-2 also substantially decreased following IS (Fig. 1G, H). Insignificant decreases in plasma level of anti-angiogenic factor angiostatin were also observed in IS patients at all timepoints compared to HVs (Fig. 1I). In contrast, the level of endostatin significantly increased during the acute and chronic phases of stroke (Fig. 1J).
Since most IS patients have at least one vascular risk factor [28], this study examined the specific correlation between the number of vascular risk factors that patients had and their plasma TNF-α levels. Observation of similar TNF-α levels in patients with and without risk factors, other than those with DM on day 7 and with TIA at baseline, refuted this hypothesis and proposed ischemic injury as the main inducer of TNF-α in stroke patients (Supplementary Fig. 3A-E). However, the presence of multiple risk factors slightly elevated TNF-α levels which reached significance when DM was one of the risk factors (Supplementary Fig. 4A-D).
Detection of higher TNF-α levels in HVs with DM, hypertension and hyperlipidemia on the other hand, proved these risk factors as the generators of TNF-α in the absence of an overt ischemic injury (Supplementary Fig. 3F-I). However, co-existence of hypertension and diabetes mellitus increased TNF-α release compared to either group alone (Supplementary Fig. 4E).
OEC-CM Protects BBB Integrity and Function Against TNF-α Injury
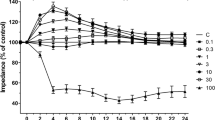
Increased TNF-α levels in patients and HVs with vascular risk factors strongly support the involvement of this cytokine in endothelial dysfunction, a prominent cause of BBB permeability [20, 29]. As cerebral edema constitutes the main cause of death after IS [7, 12], this study assessed the therapeutic potential of an emerging strategy, employing hypoxia-primed OEC-CM, in mitigating the previously reported barrier-disruptive effects of TNF-α. For this, two triple cell culture models of human BBB, consisting of astrocytes, pericytes, and HBMECs alone or mixed with OECs (Fig. 2A) were subjected to TNF-α (10 ng/ml) for 6 h in the absence or presence of OEC-CM. Reversal of TNF-α-induced decreases in TEER readings and increases in paracellular flux of NaF evinced the protective effect of OEC-CM on barrier integrity and function, respectively (Fig. 2B, C). Suppression of TNF-α-induced contractile actin bundles, otherwise known as stress fibers, leading to the formation of intercellular gaps between adjacent ECs [20, 30], also contributed to the barrier-protective role of OEC-CM (Fig. 2D, E). Scrutiny of mechanisms underlying the barrier-restorative effects of OEC-CM attributed these benefits to markedly reduced oxidative stress and apoptosis, ascertained by decreased NADPH oxidase activity, superoxide anion generation, DNA fragmentation, caspase 3/7 activity and increased cell viability in both HBMEC and OECs (Supplementary Figs. 5 and 6, respectively).
Schematic diagram of an in vitro model of human BBB and the effect of OEC-CM on BBB integrity and function and actin cytoskeleton organization in HBMECs and OECs. (A) In vitro models of human BBB consisting of astrocytes, pericytes, and HBMECs alone or mixed with OECs. (B, C) TNF-α significantly disrupted BBB integrity and function, as shown by decreases in TEER and concomitant increases in paracellular flux of sodium fluorescein, which were prevented by OEC-CM treatment. (D) Co-treatment with OEC-CM prevented the effects of TNF-α on cytoskeletal reorganization in HBMECs and OECs and decreased stress fiber formation (white arrows). (E) Quantification of stress fiber formation in both cells. Scale bar: 25 μm. *P < 0.05 versus BBB formed by HBMECs or control, #P < 0.05 versus BBB formed by HBMECs exposed to TNF-α, †P < 0.05 versus BBB formed by HBMECs exposed to TNF-α and OEC-CM, φP < 0.05 versus BBB formed by HBMECs and OECs, ψP < 0.05 versus BBB formed by HBMECs and OECs exposed to TNF-α (one-way ANOVA followed by Tukey's post-hoc analysis). BBB, blood–brain barrier; HBMECs, human brain microvascular endothelial cells; OEC-CM, outgrowth endothelial cell-derived conditioned medium; OECs, outgrowth endothelial cells; TNF-α, tumor necrosis factor-α
OEC-CM Promotes Cellular Mechanisms Required for Angiogenesis
Angiogenesis, formation of new blood vessels from pre-existing ones, is an important process in post-ischemic tissue injury and BBB remodeling and involves the migration, proliferation, and differentiation of ECs [27, 31]. It is controlled by a number of specific factors, including inflammatory cytokines and adhesion molecules [32]. Considering these and the pivotal roles of BMECs and OECs in BBB formation, this study assessed the proliferative and migratory potential of these particular cell lines in the absence and presence of OEC-CM and documented significant increases in both parameters in both cell types within 24 h of incubation using a wound scratch (Fig. 3A, B) and WST-1 assay (Supplementary Fig. 7).
The effect of OEC-CM on HBMEC and OEC functional characteristics and analysis of angiogenesis-related proteins in HBMEC and OEC secretomes and OEC-CM. (A, B) OEC-CM accelerated wound closure in both HBMEC and OECs. (C-E) OEC-CM negated the impact of TNF-α on HBMEC and OEC tubule network. (F, G) Treatments with OEC-CM neutralized the inhibitory effect of TNF-α on HBMEC and OEC adhesion to fibronectin, an extracellular matrix protein. (H, I) Proteome profiling of OEC-CM along with HBMEC and OEC secretomes revealed significant variations in various pro- and anti-angiogenic factors e.g. endothelin-1, MCP-1 and endostatin in OEC-CM. Scale bars = 100 μm. *P < 0.05 versus control, #P < 0.05 versus TNF-α (one-way ANOVA followed by Tukey's post-hoc analysis). HBMECs, human brain microvascular endothelial cells; IL-8, interleukin-8; MCP-1, monocyte chemoattractant protein-1; OEC-CM, outgrowth endothelial cell-derived conditioned medium; OECs, outgrowth endothelial cells; TIMP-1, tissue inhibitors of metalloproteinase-1; TNF-α, tumor necrosis factor-α; uPA, urokinase plasminogen activator
The formation of tubules by ECs constitutes another critical step in the angiogenic process which occurs during growth of the incipient vascular sprout and requires the synchronized functioning of cytoskeleton and cell–matrix adhesion [33,34,35]. By nullifying the disruptive effects of TNF-α on the number and length of tubes formed on Matrigel, a reliable marker for angiogenesis in vivo [36], OEC-CM may promote help revascularize the ischemic tissue (Fig. 3C-E). Similarly, OEC-CM abrogates the disruptive effects of TNF-α on cell-extracellular matrix adhesion, a prerequisite for migration, proliferation, and tubulogenic activity, as evidenced by dramatic increases in the number of HBMEC and OECs adhering to fibronectin (Fig. 3F, G) and collagen (Supplementary Fig. 8A, B), important components of the ECM, in equal measures.
Proteome profiling of OEC-CM versus HBMEC and OEC secretome in an attempt to identify the specific elements involved in aforementioned OEC-CM-mediated benefits revealed significant increases in pro-angiogenic factors, endothelin-1 (ET-1) and monocyte chemoattractant protein-1 (MCP-1) and a decrease in anti-angiogenic factor endostatin in OEC-CM (Fig. 3H, I). ET-1 stimulates the mobilization and proliferation of ECs through activation of both ETA and ETB receptors [37, 38] where specific activation of ETA receptor increases VEGF expression which in turn directly or through induction of MCP-1 promote EC migration and proliferation and participate in angiogenesis [39,40,41].
Compared to OECs, a specific increase in interleukin-8 (IL-8) and decreases in urokinase plasminogen activator (uPA), serpin-E1, angiopoietin-2 (Ang2), and pentraxin-3 levels were also found in OEC-CM. Like endothelins, IL-8 also directly enhances EC proliferation and survival [42]. Serpin-E1 or serine protease inhibitor-1 acts as the main inhibitor of uPA, an enzyme that catalyzes the conversion of plasminogen to plasmin. Attenuation of uPA in hypoxia-induced OEC-CM may maintain normal cellular phenotype and help prevent vascular leakage through regulation of MMP-2 and NADPH oxidase [43,44,45]. Similarly, Ang2 and acute phase protein pentraxin-3 also play critical roles in angiogenesis and vascular dysfunction in conditions associated with TNF-α release [46]. Observation of similar levels of thrombospondin-1 and tissue inhibitor of metalloproteinase-1 (TIMP-1) in OEC-CM and HBMEC and OEC supernatants rule out the possible involvement of these proteins in OEC-CM-mediated cell proliferation and migration (Fig. 3H, I).
Given the conspicuous differences in HV and IS patient plasma endostatin levels and the role of this anti-angiogenic factor in EPC functionality and post-stroke angiogenesis [47, 48], this study specifically assessed the impact of OEC-CM on endostatin levels in HBMEC and OEC subjected to TNF-α and significantly inhibited TNF-α-mediated elevations observed in both cell types (Supplementary Fig. 9).
Discussion
Despite introduction of various guidelines, such as ARRIVE and STEPS [49, 50] to facilitate extrapolation of pre-clinical data to clinical settings, most stroke trials have failed to replicate the favorable results observed in relevant pre-clinical studies [8]. Scrutiny of the past failures suggest that critical analysis of clinical and biochemical changes brought about by ischemic injury may help identify new therapeutic targets and inform the design of pre-clinical research [10]. Through assessment of a large set of clinical, demographic, and biochemical data obtained from the participants of the DMT EPC study, the current study has found a consistent increase in TNF-α levels across all phases of IS compared to HVs. Similar increases have also been found in SDF-1 levels during the acute, subacute, and early chronic phase of IS. As SDF-1 mediates mobilization and homing of EPCs to the site of injury, its increased release in the immediate aftermath of stroke may counter the disruptive effects of stroke on cerebrovasculature, reduce infarct volume, and improve functional outcome [51]. Because cell damage and inflammation are mostly returned to normal levels in the later stages of stroke, SDF-1 may not be required as much, explaining the gradual decreases observed in its levels during the chronic phases of stroke.
Unlike TNF-α and SDF-1, no significant variation was observed in the levels of VEGF and PDGF-BB between IS patients and HVs until day 90 post-stroke. Despite presence of reports showing dramatic increases in serum VEGF levels in IS patients [52, 53], the findings of recent studies and a meta-analysis analyzing data from 14 case–control studies concur with the findings of the present study and indicate a lack of correlation between serum VEGF or PDGF-BB levels and time of testing performed after IS [54,55,56]. Even so, future multicenter studies with bigger and heterogenous participant population may better address these points.
Due their ability to re-endothelialize damaged endothelium and promote angiogenesis, EPCs and their functional subtype OECs have recently been at the forefront of clinical investigation [12, 57]. Subsequent studies have attributed much of the vasculoreparative effects of EPCs/OECs to the trophic factors that they release [58]. Using an interactive in vitro model of human BBB, the present study has shown that treatment with hypoxia-primed OEC-CM, a promising cell-based but cell-free strategy, mitigates the disruptive effects of TNF-α on vascular integrity and function, as proven by increases in TEER readings and decreases in paracellular flux of NaF. However, the capacity of EPCs to replace the dead or damaged cerebral ECs to maintain vascular homeostasis cannot be overlooked [12, 13]. Indeed, establishment of equally functional BBB with HBMECs alone or in combination with OECs confirm the ability of OECs to interact with mature ECs.
Enhanced NADPH oxidase activity and O2.− production are involved in TNF-α-induced BBB damage [20, 29]. Observation of similar TAC in IS patients across all study timepoints indicate that pro-oxidant, rather than anti-oxidant, pathways plays a major role following cerebral ischemia. Diminished BBB damage and infarct volumes in NADPH oxidase-knockout versus wild-type mice and those treated with NADPH oxidase inhibitors (apocynin or DPI) before induction of MCAo, confirm the barrier-disruptive impact of this oxidase in IS settings [59, 60]. Inhibition of NADPH oxidase activity and O2.− production in HBMECs and OECs subjected to TNF-α with OEC-CM prove the anti-oxidant effect of this therapy. Suppression of ROS, apoptosis, and inflammatory mediator generation in ECs subjected to H2O2 by OEC-CM reinforces the modulatory role of this therapeutic regimen on pro-oxidative mechanisms [61].
Apoptosis of cerebrovascular cells induced by inflammatory mediators may also contribute to cerebral barrier damage [30]. Analyses of several markers for cell viability/death have shown significant elevations in DNA fragmentation, pro-apoptotic caspase-3/7 enzyme activities and cell death (ascertained by reduced calcein AM staining) in TNF-α-treated HBMECs and OECs. Increased TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining, capable of detecting DNA breaks in the latter phases of apoptosis, had also previously been shown in HBMECs exposed to TNF-α [30]. Co-treatment of HBMEC and OECs with OEC-CM in the current study attenuated the apoptotic effects of TNF-α, markedly increased cell viability and invariably helped maintain appropriate BBB function. Treatment of oligodendrocyte precursor cells subjected to oxygen–glucose deprivation with EPC-CM has previously been shown to inhibit apoptosis [62]. Activation of canonical NF-κB pathway that regulates expression of various genes related to cell survival, proliferation, differentiation and inflammatory responses may also be instrumental in TNF-α-evoked barrier disruption. However, treatments with EPC secretome has been shown to suppress NF-κB transcriptional activity, leading to enhanced neovascularization in vivo following stroke and myocardial ischemia and restoring BBB integrity in vitro [63,64,65].
In addition to reparative processes, regenerative processes, notably angiogenesis involving EC proliferation, tubulogenesis, branching and anastomosis also contribute to neurological recovery after IS [27]. Progressive declines in plasma level of pro-angiogenic VEGF and PDGF-BB and a concomitant increase in anti-angiogenic endostatin observed in this study appear to be the main drivers of impaired angiogenesis after IS. Furthermore, the increased tubule network dissolution and impaired proliferation concurred with endostatin overproduction in HBMECs and OECs treated with TNF-α affirmed the seminal role of this cytokine in vitiated angiogenesis [66]. Normalization of these elements by OEC-CM displayed its modulatory influence on anti-angiogenic factor synthesis/release and affirmed its reparative function against TNF-α. Increased adhesion of resident ECs and OECs to fibronectin and collagen may also be instrumental in angiogenesis-stimulatory effect of OEC-CM, as strong adhesion to extracellular matrix components is an important prerequisite for the homing of circulating EPCs [67]. Neutralization of TNF-α-mediated activation of matrix metalloproteinases by OEC-CM is likely to contribute to its pro-angiogenic effects [68]. Detailed analysis of the elements released by OECs upon hypoxic-priming implicate ET-1, MCP-1, and IL-8 in the angiogenesis-promoting function of OEC-CM. Diminished expression of uPA, serpin-1, Ang2, endostatin, and pentraxin-3 may also be responsible for hypoxia-augmented pro-angiogenic effects of OEC-CM.
There are some limitations to this study. Since 88.2% of patients had mRS score of ≤ 3 on day 90 after stroke, the correlation between TNF-α levels and disease outcome could not be ascertained. As lacunar and cortical strokes made up almost half of the overall sample size, the neurological improvements was unlikely to derive from a particular stroke subtype. Considering that treatment with an anti-TNF-< antibody negate the ischemic injury-evoked BBB damage [29], analyses of barrier-restorative capacities of aTNF-α targeting agent, OEC-CM and combination of both would have been useful. It is likely that inflammatory mediators other than TNF-α, e.g. IL-6 and IL-33, may also significantly contribute to ischemic injury-evoked cerebral barrier damage [69, 70]. The specific roles of these inflammatory mediators and crosstalk between them during/after IS deserve attention in future studies.
Conclusion
In depth analysis of plasma samples collected for The Dunhill Medical Trust EPC study has shown a constant increase in TNF-α levels in patients with IS compared to HVs. In vitro studies performed with a clinically-relevant concentration of TNF-< has proven that TNF-< compromises BBB integrity through a series of mechanisms, including EC apoptosis, oxidative stress, cytoskeletal reorganization and cell–matrix adhesion. Scrutiny of OEC-CM as an emerging cell-free therapeutic strategy has proven it to be very effective in negating the deleterious effects of TNF-< on BBB. Specific molecule(s) that contribute the most to the BBB-restorative effect of OEC-CM deserve further investigation in future studies. Similarly, it will be important to determine the therapeutic impact of OEC-CM in future in vivo studies.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Roth, G. A., Abate, D., Abate, K. H., et al. (2018). Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1736–1788. https://doi.org/10.1016/S0140-6736(18)32203-7
Kyu, H. H., Abate, D., Abate, K. H., et al. (2018). Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1859–1922. https://doi.org/10.1016/S0140-6736(18)32335-3
Nogueira, R. G., Jadhav, A. P., Haussen, D. C., et al. (2017). Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. New England Journal of Medicine, 378(1), 11–21. https://doi.org/10.1056/NEJMoa1706442
Malhotra, K., Goyal, N., Katsanos, A. H., et al. (2020). Association of blood pressure with outcomes in acute stroke thrombectomy. Hypertension, 75(3), 730–739. https://doi.org/10.1161/HYPERTENSIONAHA.119.14230
Virani, S. S., Alonso, A., Aparicio, H. J., et al. (2021). Heart disease and stroke statistics—2021 update. Circulation, 143(8), e254–e743. https://doi.org/10.1161/CIR.0000000000000950
Kadir, R. R. A., Alwjwaj, M., Bayraktutan, U. (2020). MicroRNA: An emerging predictive, diagnostic, prognostic and therapeutic strategy in ischaemic stroke. Cellular and Molecular Neurobiology. https://doi.org/10.1007/s10571-020-01028-5
Rakkar, K., & Bayraktutan, U. (2016). Increases in intracellular calcium perturb blood–brain barrier via protein kinase C-alpha and apoptosis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1862(1), 56–71. https://doi.org/10.1016/j.bbadis.2015.10.016
Chen, X., & Wang, K. (2016). The fate of medications evaluated for ischemic stroke pharmacotherapy over the period 1995–2015. Acta Pharm Sin B, 6(6), 522–530. https://doi.org/10.1016/j.apsb.2016.06.013
Lourbopoulos, A., Mourouzis, I., Xinaris, C., et al. (2021). Translational block in stroke: A constructive and “Out-of-the-Box” reappraisal. Frontiers in Neuroscience, 15, 652403–652403. https://doi.org/10.3389/fnins.2021.652403
Bix, G. J., Fraser, J. F., Mack, W. J., et al. (2018). Uncovering the rosetta stone: Report from the first annual conference on key elements in translating stroke therapeutics from pre-clinical to clinical. Translational Stroke Research, 9(3), 258–266. https://doi.org/10.1007/s12975-018-0628-9
Rakkar, K., Othman, O., Sprigg, N., et al. (2020). Endothelial progenitor cells, potential biomarkers for diagnosis and prognosis of ischemic stroke: Protocol for an observational case-control study. Neural Regeneration Research, 15(7), 1300–1307. https://doi.org/10.4103/1673-5374.269028
Bayraktutan, U. (2019). Endothelial progenitor cells: Potential novel therapeutics for ischaemic stroke. Pharmacological Research, 144, 181–191. https://doi.org/10.1016/j.phrs.2019.04.017
Abdulkadir, R. R., Alwjwaj, M., Othman, O. A., et al. (2020). Outgrowth endothelial cells form a functional cerebral barrier and restore its integrity after damage. Neural Regeneration Research, 15(6), 1071–1078. https://doi.org/10.4103/1673-5374.269029
Garbuzova-Davis, S., Haller, E., Lin, R., et al. (2017). Intravenously transplanted human bone marrow endothelial progenitor cells engraft within brain capillaries, preserve mitochondrial morphology, and display pinocytotic activity toward blood-brain barrier repair in ischemic stroke rats. Stem Cells, 35(5), 1246–1258. https://doi.org/10.1002/stem.2578
Kadir, R. R. A., Alwjwaj, M., & Bayraktutan, U. (2022). Treatment with outgrowth endothelial cells protects cerebral barrier against ischemic injury. Cytotherapy. https://doi.org/10.1016/j.jcyt.2021.11.005
Medina, R. J., O’Neill, C. L., O’Doherty, T. M., et al. (2013). Ex vivo expansion of human outgrowth endothelial cells leads to IL-8-mediated replicative senescence and impaired vasoreparative function. Stem Cells, 31(8), 1657–1668. https://doi.org/10.1002/stem.1414
Alwjwaj, M., Kadir, R. R. A., & Bayraktutan, U. (2021). The secretome of endothelial progenitor cells: A potential therapeutic strategy for ischemic stroke. Neural Regeneration Research, 16(8), 1483–1489. https://doi.org/10.4103/1673-5374.303012
Di Santo, S., Seiler, S., Fuchs, A. L., et al. (2014). The secretome of endothelial progenitor cells promotes brain endothelial cell activity through PI3-kinase and MAP-kinase. PLoS One, 9(4), e95731. https://doi.org/10.1371/journal.pone.0095731
Harrell, C. R., Fellabaum, C., Jovicic, N., et al. (2019). Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells, 8(5), 467. https://doi.org/10.3390/cells8050467
Abdullah, Z., & Bayraktutan, U. (2014). NADPH oxidase mediates TNF-α-evoked in vitro brain barrier dysfunction: Roles of apoptosis and time. Molecular and Cellular Neuroscience, 61, 72–84. https://doi.org/10.1016/j.mcn.2014.06.002
Kadir, R. R. A., Alwjwaj, M., & Bayraktutan, U. (2022). Establishment of an in vitro model of human blood-brain barrier to study the impact of ischemic injury. The Blood-Brain Barrier: Methods and Protocols, 2492, 143.
Kadir, R. R. A., Alwjwaj, M., McCarthy, Z., et al. (2021). Therapeutic hypothermia augments the restorative effects of PKC-β and Nox2 inhibition on an in vitro model of human blood–brain barrier. Metabolic Brain Disease. https://doi.org/10.1007/s11011-021-00810-8
Chevalier, F., Lavergne, M., Negroni, E., et al. (2014). Glycosaminoglycan mimetic improves enrichment and cell functions of human endothelial progenitor cell colonies. Stem Cell Research, 12(3), 703–715. https://doi.org/10.1016/j.scr.2014.03.001
Kadir, R. R. A., Alwjwaj, M., & Bayraktutan, U. (2022). Protein kinase C-β distinctly regulates blood-brain barrier-forming capacity of Brain Microvascular endothelial cells and outgrowth endothelial cells. Metabolic Brain Disease. https://doi.org/10.1007/s11011-022-01041-1
Allen, C., Srivastava, K., & Bayraktutan, U. (2010). Small GTPase RhoA and Its effector rho kinase mediate oxygen glucose deprivation-evoked in vitro cerebral barrier dysfunction. Stroke, 41(9), 2056–2063. https://doi.org/10.1161/STROKEAHA.109.574939
Allen, C. L., & Bayraktutan, U. (2009). Oxidative stress and its role in the pathogenesis of ischaemic stroke. International Journal of Stroke, 4(6), 461–470. https://doi.org/10.1111/j.1747-4949.2009.00387.x
Hatakeyama, M., Ninomiya, I., & Kanazawa, M. (2020). Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regeneration Research, 15(1), 16–19. https://doi.org/10.4103/1673-5374.264442
Allen, C. L., & Bayraktutan, U. (2008). Risk Factors for Ischaemic Stroke. International Journal of Stroke, 3(2), 105–116. https://doi.org/10.1111/j.1747-4949.2008.00187.x
Abdullah, Z., Rakkar, K., Bath, P. M. W., et al. (2015). Inhibition of TNF-α protects in vitro brain barrier from ischaemic damage. Molecular and Cellular Neuroscience, 69, 65–79. https://doi.org/10.1016/j.mcn.2015.11.003
Abdullah, Z. (1862). Bayraktutan U (2016) Suppression of PKC-α attenuates TNF-α-evoked cerebral barrier breakdown via regulations of MMP-2 and plasminogen-plasmin system. Biochimica et Biophysica Acta, 7, 1354–1366. https://doi.org/10.1016/j.bbadis.2016.03.014
Yang, Y., & Torbey, M. T. (2020). Angiogenesis and blood-brain barrier permeability in vascular remodeling after stroke. Current Neuropharmacology, 18(12), 1250–1265. https://doi.org/10.2174/1570159x18666200720173316
Lamalice, L., Le Boeuf, F., & Huot, J. (2007). Endothelial cell migration during angiogenesis. Circulation Research, 100(6), 782–794. https://doi.org/10.1161/01.RES.0000259593.07661.1e
Iruela-Arispe, M. L., & Davis, G. E. (2009). Cellular and molecular mechanisms of vascular lumen formation. Developmental Cell, 16(2), 222–231. https://doi.org/10.1016/j.devcel.2009.01.013
Michalski, D., Spielvogel, E., Puchta, J., et al. (2020). Increased immunosignals of collagen IV and fibronectin indicate ischemic consequences for the neurovascular matrix adhesion zone in various animal models and human stroke tissue. Frontiers in Physiology, 11, 575598. https://doi.org/10.3389/fphys.2020.575598
Mongiat, M., Andreuzzi, E., Tarticchio, G., et al. (2016). Extracellular matrix, a hard player in angiogenesis. International Journal of Molecular Sciences, 17(11), 1822. https://doi.org/10.3390/ijms17111822
Aref, Z., & Quax, P. H. A. (2021). In vivo matrigel plug assay as a potent method to investigate specific individual contribution of angiogenesis to blood flow recovery in mice. International Journal of Molecular Sciences, 22(16), 8909. https://doi.org/10.3390/ijms22168909
Morbidelli, L., Orlando, C., Maggi, C. A., et al. (1995). Proliferation and migration of endothelial cells is promoted by endothelins via activation of ETB receptors. American Journal of Physiology-Heart and Circulatory Physiology, 269(2), H686–H695. https://doi.org/10.1152/ajpheart.1995.269.2.H686
Dong, F., Zhang, X., Wold, L. E., et al. (2005). Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: Role of ETB receptor, NADPH oxidase and caveolin-1. British Journal of Pharmacology, 145(3), 323–333. https://doi.org/10.1038/sj.bjp.0706193
Yamada, M., Kim, S., Egashira, K., et al. (2003). Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arteriosclerosis, Thrombosis, and Vascular Biology, 23(11), 1996–2001. https://doi.org/10.1161/01.ATV.0000096208.80992.63
Matsuura, A., Yamochi, W., Hirata, K.-i, et al. (1998). Stimulatory interaction between vascular endothelial growth factor and endothelin-1 on each gene expression. Hypertension, 32(1), 89–95. https://doi.org/10.1161/01.HYP.32.1.89
Wu, M. H., Huang, C. Y., Lin, J. A., et al. (2014). Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene, 33(13), 1725–1735. https://doi.org/10.1038/onc.2013.109
Li, A., Dubey, S., Varney, M. L., et al. (2003). IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. The Journal of Immunology, 170(6), 3369. https://doi.org/10.4049/jimmunol.170.6.3369
Kadir, R. R. A., & Bayraktutan, U. (2020). Urokinase plasminogen activator: A potential thrombolytic agent for ischaemic stroke. Cellular and Molecular Neurobiology, 40(3), 347–355. https://doi.org/10.1007/s10571-019-00737-w
Rakkar, K., Srivastava, K., & Bayraktutan, U. (2014). Attenuation of urokinase activity during experimental ischaemia protects the cerebral barrier from damage through regulation of matrix metalloproteinase-2 and NAD(P)H oxidase. European Journal of Neuroscience, 39(12), 2119–2128. https://doi.org/10.1111/ejn.12552
Bayraktutan, U., & Jones, P. (1995). Expression of the human gene encoding urokinase plasminogen activator receptor is activated by disruption of the cytoskeleton. Experimental Cell Research, 221(2), 486–495. https://doi.org/10.1006/excr.1995.1400
Akwii, R. G., Sajib, M. S., Zahra, F. T., et al. (2019). Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells, 8(5), 471. https://doi.org/10.3390/cells8050471
Schuch, G., Heymach, J. V., Nomi, M., et al. (2003). Endostatin inhibits the vascular endothelial growth factor-induced mobilization of endothelial progenitor cells. Cancer Research, 63(23), 8345–8350.
Malik, A. R., Lips, J., Gorniak-Walas, M., et al. (2020). SorCS2 facilitates release of endostatin from astrocytes and controls post-stroke angiogenesis. Glia, 68(6), 1304–1316. https://doi.org/10.1002/glia.23778
Percie du Sert, N., Hurst, V., Ahluwalia, A., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Veterinary Research, 16(1), 242–242. https://doi.org/10.1186/s12917-020-02451-y
Boltze, J., Modo, M. M., Mays, R. W., et al. (2019). Stem cells as an emerging paradigm in stroke 4. Stroke, 50(11), 3299–3306. https://doi.org/10.1161/STROKEAHA.119.025436
Kukumberg, M., Zaw, A. M., Wong, D. H. C., et al. (2020). Characterization and functional assessment of endothelial progenitor cells in ischemic stroke patients. Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-020-10064-z
Slevin, M., Krupinski, J., Slowik, A., et al. (2000). Serial measurement of vascular endothelial growth factor and transforming growth factor-β1 in serum of patients with acute ischemic stroke. Stroke, 31(8), 1863–1870. https://doi.org/10.1161/01.STR.31.8.1863
Prodjohardjono, A., Vidyanti, A. N., Susianti, N. A., et al. (2020). Higher level of acute serum VEGF and larger infarct volume are more frequently associated with post-stroke cognitive impairment. PLoS One, 15(10), e0239370. https://doi.org/10.1371/journal.pone.0239370
Seidkhani-Nahal, A., Khosravi, A., Mirzaei, A., et al. (2021). Serum vascular endothelial growth factor (VEGF) levels in ischemic stroke patients: A systematic review and meta-analysis of case–control studies. Neurological Sciences, 42(5), 1811–1820. https://doi.org/10.1007/s10072-020-04698-7
Huţanu, A., Iancu, M., Maier, S., et al. (2020). Plasma biomarkers as potential predictors of functional dependence in daily life activities after ischemic stroke: A single center study. Annals of Indian Academy of Neurology, 23(4), 496–503. https://doi.org/10.4103/aian.AIAN_74_19
Kim, K. J., Jeong, S. W., Ryu, W. S., et al. (2021). Platelet-derived growth factor is associated with progression of symptomatic intracranial atherosclerotic stenosis. Journal of Clinical Neurology, 17(1), 70–76. https://doi.org/10.3988/jcn.2021.17.1.70
Bayraktutan, U. (2018). Endothelium, endothelial progenitor cells and stroke. Journal of Neurology and Clinical Neuroscience, 2(2), 02–03.
Maki, T., Morancho, A., Martinez-San Segundo, P., et al. (2018). Endothelial progenitor cell secretome and oligovascular repair in a mouse model of prolonged cerebral hypoperfusion. Stroke, 49(4), 1003–1010. https://doi.org/10.1161/strokeaha.117.019346
Nagel, S., Genius, J., Heiland, S., et al. (2007). Diphenyleneiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral ischemia of the rat. Brain Research, 1132(1), 210–217. https://doi.org/10.1016/j.brainres.2006.11.023
Tang, X. N., Cairns, B., Cairns, N., et al. (2008). Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience, 154(2), 556–562. https://doi.org/10.1016/j.neuroscience.2008.03.090
Yang, Z., von Ballmoos, M. W., Faessler, D., et al. (2010). Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis, 211(1), 103–109. https://doi.org/10.1016/j.atherosclerosis.2010.02.022
Zhou, N., Wang, L., Fu, P., et al. (2021). Conditioned medium-preconditioned EPCs enhanced the ability in oligovascular repair in cerebral ischemia neonatal rats. Stem Cell Research & Therapy, 12(1), 118. https://doi.org/10.1186/s13287-021-02157-4
Chen, J., Wang, X., Hu, J., et al. (2021). FGF20 protected against BBB disruption after traumatic brain injury by upregulating junction protein expression and inhibiting the inflammatory response. Frontiers in Pharmacology, 11, 590669–590669. https://doi.org/10.3389/fphar.2020.590669
Yue, Y., Wang, C., Benedict, C., et al. (2020). Interleukin-10 deficiency alters endothelial progenitor cell-derived exosome reparative effect on myocardial repair via integrin-linked kinase enrichment. Circulation Research, 126(3), 315–329. https://doi.org/10.1161/CIRCRESAHA.119.315829
Zhang, C., Wang, J., Ma, X., et al. (2018). ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. Journal of Cellular and Molecular Medicine, 22(3), 1873–1882. https://doi.org/10.1111/jcmm.13471
Dixelius, J., Larsson, H., Sasaki, T., et al. (2000). Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood, 95(11), 3403–3411.
Jiang, C., Li, R., Ma, X., et al. (2020). AMD3100 and SDF-1 regulate cellular functions of endothelial progenitor cells and accelerate endothelial regeneration in a rat carotid artery injury model. Molecular Medicine Reports, 22(4), 3201–3212. https://doi.org/10.3892/mmr.2020.11432
Brauer, R., Beck, I. M., Roderfeld, M., et al. (2011). Matrix metalloproteinase-19 inhibits growth of endothelial cells by generating angiostatin-like fragments from plasminogen. BMC Biochemistry, 12(1), 38. https://doi.org/10.1186/1471-2091-12-38
Qian, L., Yuanshao, L., Wensi, H., et al. (2016). Serum IL-33 is a novel diagnostic and prognostic biomarker in acute ischemic stroke. Aging & Disease, 7(5), 614–622. https://doi.org/10.14336/AD.2016.0207
Basic Kes, V., Simundic, A.-M., Nikolac, N., et al. (2008). Pro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clinical Biochemistry, 41(16), 1330–1334. https://doi.org/10.1016/j.clinbiochem.2008.08.080
Funding
This work is, in part, supported by a grant from The Dunhill Medical Trust (R459/0216).
Author information
Authors and Affiliations
Contributions
RRAK and MA performed the experimental study. OAO and KR conducted the clinical study. RRAK analysed the data and wrote the original draft of the manuscript. NS and PB were the DMT grant co-applicants and performed manuscript revision. UB was the chief investigator of the DMT study. He designed the current study, interpreted the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The Dunhill Medical Trust EPC study protocol was reviewed and approved by West Midlands—Coventry & Warwickshire Research Ethics Committee (16/WM/0304).
Consent to Participate
All study participants provided written informed consent prior to enrolment.
Consent for Publication
This manuscript has been approved by all authors and is solely the work of the authors named.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadir, R.R.A., Alwjwaj, M., Rakkar, K. et al. Outgrowth Endothelial Cell Conditioned Medium Negates TNF-α-Evoked Cerebral Barrier Damage: A Reverse Translational Research to Explore Mechanisms. Stem Cell Rev and Rep 19, 503–515 (2023). https://doi.org/10.1007/s12015-022-10439-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10439-4