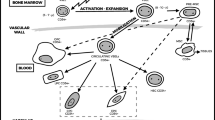
Abstract
Mesenchymal stem cell (MSC) therapy has gained significant traction in the context of cardiovascular repair, and have been proposed to exert their regenerative effects via the secretion of paracrine factors. In this systematic review, we examined the literature and consolidated available evidence for the “paracrine hypothesis”. Two Ovid SP databases were searched using a strategy encompassing paracrine mediated MSC therapy in the context of ischemic heart disease. This yielded 86 articles which met the selection criteria for inclusion in this study. We found that the MSCs utilized in these articles were primarily derived from bone marrow, cardiac tissue, and adipose tissue. We identified 234 individual protective factors across these studies, including VEGF, HGF, and FGF2; which are proposed to exert their effects in a paracrine manner. The data collated in this systematic review identifies secreted paracrine factors that could decrease apoptosis, and increase angiogenesis, cell proliferation, and cell viability. These included studies have also demonstrated that the administration of MSCs and indirectly, their secreted factors can reduce infarct size, and improve left ventricular ejection fraction, contractility, compliance, and vessel density. Furthering our understanding of the way these factors mediate repair could lead to the identification of therapeutic targets for cardiac regeneration.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adult mammalian heart exhibits limited capacity for cellular regeneration, thus injuries causing myocyte loss such as a myocardial infarction (MI) result in the activation of pro-fibrotic pathways that initiate healing following a cardiac insult but also lead to irreversible scarring. Long-term activation of these pathways results in ventricular stiffness, contractile dysfunction, and cellular hypertrophy and apoptosis. Ultimately, these pathological changes severely impair physiological functioning of the heart, and lead to the irreversible development of heart failure, for which therapeutic options are currently limited.
Stem cell therapy has emerged as a promising approach to repair the damaged myocardium, with the aim of providing the infarcted heart with an exogenous supply of regenerative elements to promote cytoprotection, vascularization, or cardiomyogenesis [1]. In particular, there has been a focus on cells of mesenchymal origin (mesenchymal stem cells – MSCs), including bone marrow derived MSCs (BM-MSCs) and cardiac progenitor cells (CPCs). Several populations of resident CPCs have been identified including c-kit+, Sca-1+, Islet 1+, and cardiospheres, all of which have promoted cardiac repair to varying degrees [2, 3]. These cell populations are cardiac lineage committed, and may offer a significant advantage when compared to their counterparts. However, given their limited numbers in the heart, they do not adequately promote cardiac repair following an acute injury independently. Nonetheless, treatment with BM-MSCs [4] and CPCs [5] in pre-clinical studies has resulted in improvements in left ventricular ejection fraction (LVEF), contractility, increased angiogenesis, and reduced infarct size. In vitro, these cells have demonstrated a capacity to differentiate into cardiomyocytes and vascular endothelial cells [5, 6], but there is no clear evidence of differentiation in vivo either pre-clinically or clinically [7]. Furthermore, studies have consistently shown that implanted BM-MSCs [8] and CPCs [9] engraft efficiently or do not survive longer than 3 weeks post-injection, suggesting that differentiation is unlikely to be the primary mechanism driving the observed improvements in cardiac outcomes. The secretion of soluble paracrine factors has been proposed as an alternative mechanism and this is termed the “paracrine hypothesis”.
Stem cells condition culture media by producing and secreting a range of cytokines, chemokines, and growth factors in their culture media. In support of the paracrine hypothesis, numerous studies have demonstrated that conditioned media alone has a similar protective effect to whole cell therapy in vitro [10,11,12,13] and in vivo [12], including promotion of cell survival and proliferation, immunomodulation, cardiac remodelling, neovascularization, and activation of resident CPC populations [14,15,16]. Some soluble factors known to be produced and released by adult stem cells include VEGF, FGF2, HGF, IGF1, IL1β, IL15, PDGF, and SDF1, [11, 12, 17]. The available literature has also identified the release of exosomes and extracellular vesicles by stem cells. The study of these vesicles is multifaceted in its nature given the complexity of characteristics, functions, and biological processes associated with them. Given they are an additional cargo packaging a range of bioactive factors such miRNAs, mRNA molecules, peptides, proteins, cytokine, and lipids, they would warrant an in depth analysis of their own right [18, 19]. For this reason, and in the interest of presenting a concise body of work, we have focused exclusively on factors shown to be directly released by stem cells of mesenchymal origin.
Despite stem cells being capable of exerting cardioprotective effects as a whole, the molecular mechanisms underpinning the release and action of individual factors vary. Consolidating factors known to be directly secreted by MSCs thus far would be beneficial as their application may circumvent the need for whole cell therapy, which possesses numerous problems including the cost and time to grow and deliver cells, donor matching, immune rejection, and the ethical and legal concerns associated with each of the potential cell types. Studies are already investigating the targeted delivery of specific factors such as HGF, IL15, and VEGF and have shown some reductions in scar size, and attenuated signs of cardiac remodelling to a certain extent in pre-clinical models of MI [20, 21]. Whilst promising, it is likely that a combination of factors would more successfully promote cardiac repair following an acute injury and numerous repair mechanisms would need to act in concert to allow recovery.
The aim of this systematic review is to consolidate the existing literature and identify paracrine factors directly released by MSCs, which may improve cardiac healing. Where available, data concerning their functional effects in vitro, in vivo, or ex vivo was extracted. In this review, we have identified a range of stem cells of mesenchymal origin, including MSCs derived from adipose tissue (AD-MSCs, APCs), bone marrow (BM-MSCs), cardiac tissue (CPCs, CSCs), menstrual blood (En-MSCs), placenta (P-MSCs), peripheral blood (PB-MSCs), and umbilical cord blood (UCB-MSCs). Throughout this article, the term MSCs will be broadly used to refer to these cell types as a whole.
Methods
Search Strategy
A systematic literature search was conducted using Ovid SP databases (Embase and Medline), and included all relevant publications to the 22 February 2022. The search strategy used for Embase and Medline are outlined in the supplementary information Tables 1 and 2 respectively. Upon completion of the search, duplicate texts were removed, uploaded to Covidence, and the titles and abstracts of the remaining articles examined for relevance to the review topic. Those that did not fit the inclusion criteria were noted, but not analyzed further. PROSPERO systematic review database registration: CRD42019127475. During the full text screening and data extraction process it became clear that the proposed quality assessment tools in our PROSPERO protocol would not be sufficient to investigate the question at hand, and thus we designed a checklist (detailed below) to better address the question at hand.
Inclusion Criteria
Retrieved texts were screened for relevance based on the inclusion criteria detailed below. Original research articles were included if they met the primary aim of identifying paracrine factors directly released by MSCs which may be capable of mediating improvements in a cardiac context. In vitro studies were included if they: 1) clearly identified the mesenchymal origin of cell type used, 2) identified protective factors released directly by MSCs thought to be behaving in a paracrine manner in the study, and 3) included of appropriate control groups in the study design. Where included studies contained relevant ex vivo or in vivo cardiac models, the reported functional associations of stem cell therapy were additionally summarized. All searches were limited to English-language articles published by 22 February 2022.
Exclusion Criteria
Review articles, conference proceedings and retracted studies were excluded from this systematic review. This review focuses on identifying paracrine factors directly released by cells of mesenchymal origin. As such, studies which: 1) used cells of non-mesenchymal origin, 2) did not directly demonstrate release of paracrine factors by cell types being investigated, or identified particles such as extracellular vesicles or exosomes, 3) investigated the protective effects of treating MSCs without appropriate controls, or 4) investigated the protective effects of culturing MSCs on biomaterials without appropriate controls were excluded from this review.
Study Selection
Three investigators (N.S.M., L.R., and J.L.) independently evaluated the titles and abstracts (n = 4443) of the identified articles according to the selection criteria, those articles of potential relevance were allocated to the next stage to be reviewed in full (n = 275). Three investigators (N.S.M., L.R., and A.J.B.) independently undertook full text screening according to the inclusion and exclusion criteria outlined above. In cases of initial disagreement on an article’s eligibility, a decision was rendered following discussion leading to consensus between investigators. Initial agreement between investigators on the eligibility of an article was assessed using percentage agreement and the kappa statistic.
Data Extraction and Quality Assessment
The following data were extracted from included studies: first author, year of publication, origin of MSCs, phenotyping of MSCs, study design, identified paracrine factors, and method used to identify paracrine factors. In studies where MSCs were treated, transfected, or cultured on biomaterials only data from appropriate control groups were considered for analysis. Data regarding in vitro, ex vivo or in vivo models of cardiac ischemia were additionally extracted. We developed a 9-point checklist (Table 1) to assess the quality of reporting and overall study design.
Results
Selection of Studies
Of the initial 4492 studies identified, 49 were identified as duplicates. Following title and abstract screening of the remaining 4443 articles, 276 were selected for full text screening, and 1 was manually included (conference abstract identified in original literature search had further associated full text publication). Of these, 190 studies were excluded primarily because they did not meet the inclusion criteria, or contained characteristics of the exclusion criteria; including not meeting study design criteria (79), use of non-mesenchymal cells (27), no protective factors identified (35), extracellular vesicles or exosomes identified (3), or study was not of cardiovascular context (6). A number of studies were excluded for retraction (1), poor quality (2), duplication (3), conference abstracts (28), literature reviews (1), or inaccessible full text (5), and a further duplicate study was excluded manually following screening in Covidence. A final total of 86 original articles were included in this review (Fig. 1). The percentage of agreement on study inclusion was 87%, and the kappa score was 0.687; signifying substantial initial agreement.
Flow diagram of systematic review search and screening results. The initial search strategy yielded 4492 references across two databases. Duplicate removal resulted in 4443 studies for title and abstract screening by two independent reviewers. 276 studies went forward to full text screening, and resulted in 86 studies for inclusion in this review
Study Characteristics & Quality Assessment
The stem cells used in these studies were primarily derived from bone marrow (59/86), cardiac tissue (16/86), and adipose tissue (11/86). Other sample sources included bone fragments (1/86), cortical bone (1/86), blood: umbilical cord blood (2/86), peripheral blood (1/86), menstrual blood (1/86), or healthy term placenta (1/86) (Fig. 2A). These samples were collected from human (31/86), rat (27/86), mouse (28/86), pig (1/86) or horse (1/86) subjects. A further study did not disclose the species the stem cells were derived from. Of the 86 articles included in this study based on identification of MSC paracrine factors, 35/86 further investigated the beneficial effects of stem cells in vitro. The functional effects of stem cell therapy were further assessed in 11/86 studies using ex vivo models of cardiac ischemia and in 44/86 using in vivo models of cardiac ischemia.
Commonly identified stem cell sources, their secreted paracrine factors, and associated molecular functions. (a) Primary sources stem cells of mesenchymal origin were derived from included bone marrow, cardiac tissue, adipose tissue, and blood. (b) The top 15 protective paracrine factors found to be secreted from cells of mesenchymal origin (c) The top 10 molecular functions of secreted factors. (a) Was created with BioRender.com
Within our quality assessment, we investigated the extent to which each of the included studies adhered to the International Society for Cellular Therapy (ISCT) proposed set of standards for identifying cells of mesenchymal origin [22] (Table 2). We found that only one of the studies met all recommended ISCT criteria in full. Adherence to plastic was reported by 55/86 studies, surface antigen expression was investigated by 62/86 studies, however these typically included a range of markers besides those recommended by the ISCT, and multipotency was reported by 38/86 studies. Only 11/86 studies scored higher than 80% in the quality assessment questionnaire. The results of the quality assessments for each article from both independent reviewers are detailed in supplementary information Table 3.
In Vitro – Commonly Identified Factors and their Effects
Across the 86 included articles, a total of 234 different factors were identified using a range of techniques including ELISA, qPCR, western blot, immunostaining, mass spectrometry, immunoassays, and microarrays.
The most commonly identified factors (Fig. 2B) directly released by MSCs were VEGF (67/86), hepatocyte growth factor (HGF, 30/86), fibroblast growth factor 2 (FGF2, 22/86), interleukin-6 (IL6, 21/86), stromal cell-derived factor 1 (SDF1, 20/86), insulin like growth factor 1 (IGF1, 18/86), C–C motif chemokine 2 (MCP1/CCL2, 13/86), interleukin-8 (IL8, 10/86), tumour necrosis factor alpha (TNFα, 9/86), interleukin-1β (IL1β, 7/86), C–C motif chemokine 5 (CCL5, 6/86), epidermal growth factor (EGF, 6/86), metalloproteinase inhibitor 1 (TIMP1, 6/86), macrophage colony-stimulating factor 1 (CSF1, 6/86), and stem cell factor (SCF, 5/86). When categorized by molecular function (Fig. 2C), the identified factors were commonly classified as growth factors, cytokines, chemokines, receptors, and hormones.
The beneficial effects of factors released by stem cells in vitro were investigated in 38/86 studies by utilizing primary adult cardiomyocytes (CMs) (5/38), primary neonatal rat cardiomyocytes (NRCs) (12/38), CM cell lines (HL-1, H9c2, AC16) (8/38), or endothelial cell lines (hDMECs, HUVECs, HMEC-1) (17/38). These cells were co-cultured with stem cells or their conditioned media under normoxic or hypoxic conditions, and the effects on angiogenesis, apoptosis, and proliferation studied when compared to appropriate controls (e.g. untreated, vehicle treated, or non-reparative cell type treated groups). The articles included in this study demonstrated that factors released by stem cells of mesenchymal origin (including human AD-MSCs, BM-MSCs, CSCs, En-MSCs, P-MSCs, and UCB-MSCs, as well as rat and mouse BM-MSCs) can reduce CM and endothelial cell apoptosis under hypoxic conditions, promote tube formation in endothelial cells, and increase endothelial cell proliferation or migration as further detailed in Table 3.
Ex Vivo and In Vivo Cardiac Models—Functional Associations of Stem Cell Therapy
Of the articles included in this study, 11/86 performed ex vivo experiments largely comprising of Langendorff experimental models of ischemia/ reperfusion (I/R) injury; and 45/86 performed in vivo experiments in which MI was induced using permanent or transient ligation of the left anterior descending (LAD) artery.
For ex vivo experiments, BM-MSCs, CSCs, or their conditioned media were perfused pre- or post-I/R injury, and resulted in overall improvements in cardiac function including increased left ventricular developed pressure (LVDP), right ventricular developed pressure (RVDP), contractility, and compliance, and reduced end diastolic pressure (EDP) during Langendorff perfusion, when compared to appropriate controls (e.g. untreated, vehicle treated, or non-reparative cell type treated groups).
For in vivo experiments, AD-MSCs, APCs, BM-MSCs, CBSCs, CPCs, CSCs, En-MSCs, and P-MSCs derived from human, rat, or mouse were utilized as whole cell or conditioned media therapy. The broad range of stem cells of mesenchymal origin studied in the included articles resulted in a range of functional improvements as measured by echocardiography or haemodynamics when compared to appropriate controls (e.g. untreated, vehicle treated, or non-reparative cell type treated groups). Treated hearts had decreased infarct size, reduced signs of cardiac remodelling, improvements in systolic and diastolic function, and reduced fibrosis. Other signs of improvement in cardiac function reported included increased vascular density and reduced CM apoptosis. Specific results of both ex vivo and in vivo experiments are expanded upon in Table 4.
Discussion
In this systematic review, we have identified 234 factors that are directly released by MSCs. These factors potentially mediate improvements in cardiac outcomes in a paracrine fashion. Our review consolidates a considerable amount of evidence for the paracrine hypothesis, and demonstrates the potential beneficial effects of these factors in cardiac models of ischemia using a variety of in vitro, ex vivo, and in vivo experimental models. Furthermore, our quality assessment criteria enabled the identification of several aspects of study design that could be improved upon within the field.
The articles included in this study isolated MSCs from a broad range of sources derived from human, rat, mouse, or horse samples. These samples included bone marrow, cardiac tissue, adipose tissue, blood (peripheral, menstrual, and umbilical cord blood), and placenta. Investigators utilized a range of methods to identify the paracrine factors as detailed in Table 3, with the most common experimental approach being to culture the stem cells of interest for a few days and collect the supernatant or conditioned media of these cells. This conditioned media was then analyzed using experimental techniques such as ELISA, qPCR, western blot, immunostaining, mass spectrometry, immunoassays, and microarrays. Given the range of experimental methods used, comparisons made, controls used, and normalization approaches taken, we determined that it was not possible to quantitatively compare the available data. Thus we determined that the meta-analysis originally proposed in our PROSPERO submission would not be possible with the reported data. Rather, we provide a comprehensive list of the paracrine factors identified, without direct comparison between studies.
Quality assessment criteria are typically designed for evaluation of randomized clinical trials, and are thus unsuitable for evaluating in vitro studies that include a broad range of experimental design and methodologies. Therefore, we developed a 9-point checklist to assess the quality of reporting and overall design of the articles included in this systematic review. According to our quality assessment checklist only 11/86 studies were deemed to be of high quality (score of 80% or higher) including whether key aspects of study design such as cell passage or number, replicates, and appropriate controls were reported, or if the minimum criteria established by the ISCT [22] were met. Only one of the studies in this systematic review adhered completely to the set of standards proposed for identifying MSCs by the ISCT. Our quality assessment highlighted the fact that there is much variance in the methods used to derive and phenotype MSCs, the extent of reporting of these methods, as well as the approaches undertaken to identify released paracrine factors. Future studies should consider paying attention to the phenotyping profile recommended by the ISCT as a means of ensuring some level of standardization across the field, to promote reproducibility and reliability of acquired data. It would also be beneficial to consider adopting common nomenclature, and clearly reporting cell passage, the number of cells used therapeutically (whether in vitro, ex vivo, or in vivo), and sample size in order to prevent bias or the reporting of false positive results.
The factors identified in this study can be broadly classified as growth factors, cytokines, chemokines, hormones, enzymes, enzymatic inhibitors, receptors, or a range of protein classes including glycoproteins, binding proteins, and extracellular matrix proteins, amongst others (Fig. 2C). These factors have been implicated in functions such as angiogenesis, cytoprotection, and cell migration and proliferation [14, 16, 101]. Whilst the distinction was not specifically made in the studies included in this systematic review, it is important to acknowledge that the release of cargo from exosomes or extracellular vesicles could have unwittingly contributed to the quantified secretome. We found that MSCs or their conditioned media had anti-apoptotic, proliferative, and migratory effects on cardiomyocytes [1, 13, 15, 27, 29, 36, 38, 44, 47, 68, 70, 79, 97, 99] and endothelial cells (ECs) [13, 72, 85, 90, 91] under normoxic or hypoxic conditions in vitro. Furthermore MSCs or their conditioned media could induce tube formation in ECs [13, 15, 27, 51, 72, 85, 90, 91, 98], demonstrating their angiogenic properties.
Whilst studies have demonstrated that conditioned media of MSCs could be equally beneficial as whole cell therapy in various models of ischemic cardiac injury [10,11,12,13, 40], the manner in which whole cell therapy versus conditioned media therapy propagates its beneficial effects are likely to vary. MSCs delivered directly as a therapeutic option, would not only offload their cargo of paracrine factors, but further communicate with resident cardiac cells to promote further release of beneficial factors, or engage cell recruitment. For example the administration of cardiac adipose tissue derived MSCs induced a shift in macrophage phenotype from a pro-inflammatory M1 profile to an immunosuppressive and reparative M2 profile. This shift in macrophage polarization was also associated with changes to the profile of cytokine secretion [24]. Identifying means to control this shift could aid in the control and resolution of inflammation following a cardiac insult.
Further evidence for cellular crosstalk is available in in vitro studies where MSCs co-cultured with CMs induced changes in the secretion levels of various paracrine factors including VEGF, HGF, and SDF-1α [25]. Moreover, conditioned media collected from these co-cultures could enhance the protective effects of MSCs [25] and increase CM proliferation [68]. MSC co-culture with ECs promoted the formation of cell aggregation structures, which is indicative of their commitment to pre-vascularization, additionally co-culture resulted transcriptomic changes in MSCs and ECs, and altered their secretory profile of IL1β and IL6 [54].
Angoulvant et al. additionally compared the effects of MSCs that were freshly suspended in growth media to MSC conditioned media therapy, and demonstrated that freshly resuspended MSCs did not produce significant levels of growth factors, however they still afforded cardioprotection in an ex vivo model of I/R injury by reducing CM cell death. Thus suggesting that MSCs may be capable of protecting CMs via cell-to-cell communication or via secretion of growth factors once contact has been made with CMs [10].
These data suggest that the manner in which whole cell therapy versus conditioned media therapy modulates the micro-environment and facilitates cellular crosstalk, and thus further release of paracrine factors varies significantly. However, given the problems associated with whole cell therapy including cost, time to grow and deliver cells, donor matching, immune rejection, and the ethical and legal concerns associated with various stem cell types, using factors secreted by these cells instead may be a more logistically viable route. This would circumvent the traditional problems associated with whole cell therapy and provide a more readily accessible therapeutic product.
The most commonly identified factor, VEGF, was found in 62/81 of the included studies, and has been investigated extensively for its therapeutic effects in vitro and in vivo. It has been shown to play a role in improving cardiac function, reducing fibrosis, and promoting angiogenesis and cell proliferation [20, 35]. It is a central growth and survival factor in the injured heart [24, 33]; with Markel et al. demonstrated it is essential for BM-MSC mediated cardioprotection by inducing a VEGF knockdown [62]. However, in contrast, another study showed that culturing MSCs in the presence of VEGF neutralising antibodies, did not diminish the protective capacity of MSC conditioned media [10]. HGF was the second most abundantly identified protective factor (25/70), and is known to exert anti-apoptotic, pro-angiogenic, and pro-migratory effects on a range of cells. Moreover, when directly delivered in a rat model of MI resulted in improved cardiac function, and reduced infarct size [21, 38, 47]. Furthermore, a study in which endogenous HGF was neutralized and subsequently restored led to the attenuation of I/R injury and protected cardiomyocytes from cell death [102]. It seems likely that the protective effects of stem cell secretion are due to multiple secreted components, rather than one specific factor given these studies demonstrated cardioprotection despite targeted neutralization of VEGF and HGF, and that multiple potential beneficial factors were consistently identified across the studies included in this review, it seems likely that the protective effects of stem cell secretion are due to multiple secreted components and context dependent, rather than one specific factor being present irrespective of injury and timing.
It is worth noting that although this review included studies for identification of beneficial factors, two studies were identified which also determined that IL-1β and CXCL12 (also known as SDF1) were non-protective secreted factors [21, 103]. IL-1β is a cytokine that plays a key role in inflammatory processes in cardiac disease, it increases significantly in the myocardium in response to an acute ischemic event; and in the context of cardiac repair has contradictory implications. Toldo et al. demonstrated that anti IL-1β therapy in a mouse model of MI prevented deterioration of overall cardiac function and cardiomyocyte cell death [104]. Moreover, in the clinical CANTOS trial, targeting IL-1β with a therapeutic antibody, Canakinumab, significantly reduced high sensitivity C-reactive protein and IL-6 levels, and led to an overall reduction in rate of recurrence of cardiovascular events [105]. Thus suggesting that anti IL-1β therapy improves overall cardiovascular outcomes of MI patients. However, 6/81 included studies proposed IL-1β to be a potentially protective factor secreted by MSCs. This suggests that the effects of IL-1β are context (type of injury, timing, cellular-source) dependent. For example, Avolio et al. specifically determined that IL-1β is abundant in the secretome of CSCs isolated from failing hearts, and has no anti-apoptotic effects in an in vitro model of I/R. Whereas CSCs derived from healthy donor hearts did [103]. They further determined that pre-incubation of failing heart CSCs with an IL-1β neutralising antibody could restore their anti-apoptotic properties. Thus demonstrating that IL-1β secretion by failing heart CSCs abolishes the protective effects of the CSC secretome. CXCL12/SDF-1 is a chemokine implicated in cardiogenesis, and recruitment of endothelial progenitor cells and other stem cells to sites of ischemic damage [3, 21]. Although we identified one study that suggested CXCL12/SDF-1 to be non-protective, the majority of articles included in the present study (18/81) identified CXCL12/SDF-1 as a potentially beneficial factor secreted by MSCs. For example, Huang et al. demonstrated that downregulating SDF-1 expression in CSCs completely abolished the beneficial effects of CSCs on cardiac function. Furthermore, blocking the SDF-1 receptor in the heart significantly attenuated the beneficial effects of CSCs in an ex vivo model of I/R injury [3]. Thus demonstrating that SDF-1 is a key factor via which this particular population of CSCs exert their effects.
The functional benefits of MSC therapy ex vivo or in vivo were investigated in 52/81 of the included studies. The dominant model used in ex vivo studies was the Langendorff based I/R injury model. These studies identified HGF, IGF-1, IL-10, TNFα, SDF-1, and VEGF as being secreted by BM-MSCs [3, 10, 33, 40, 41, 43, 48, 60, 62, 73] or CSCs [3] in their conditioned media. The majority of these studies perfused BM-MSCs or CSCs as whole cell therapy [3, 33, 40, 41, 43, 48, 60, 62, 73]. The improvements in infarct size and cardiac function reported in each were subsequently attributed to the paracrine factors released by MSCs, although causative data was not always present. Two studies, however, did investigate a causal link by perfusing the conditioned media of BM-MSCs [10] or CSCs [3] in their experimental model. The first demonstrated that the conditioned media of BM-MSCs was equally effective at reducing cardiac injury as BM-MSCs in both in vitro and ex vivo simulated ischemia models [10]. Furthermore, Huang et al., identified SDF-1 as being a highly abundant paracrine factor secreted by CSCs. They determined that the paracrine factors of CSCs mediated cardioprotection when delivered pre-I/R [3]. Whilst it is important to note that ex vivo experimental methods cannot recapitulate the recruitment of various cell types including immune cells to the heart and investigate their dynamic interaction; experiments utilizing conditioned media are able to test a causal relationship between the factors released by MSCs and observed improvements in cardiac outcomes.
Similar patterns were present in the in vivo experiments conducted within the included articles. Investigators commonly injected MSCs intramuscularly or intravenously at varying periods following permanent or transient induction of MI. Conditioned media was only delivered in four of the included studies utilizing MI models [13, 15, 29, 79]. These studies demonstrated that the conditioned media of MSCs derived from adipose tissue [29], bone marrow [13, 79], and placenta [15], could protect CMs from cell death under hypoxic conditions [13, 15, 29, 79]. Furthermore, utilizing the conditioned media therapeutically in in vivo models of MI improved systolic and diastolic function, reduced overall infarct size, prevented cell death in the infarcted area, and increased vessel density when compared to control media [13, 15, 29, 79]. The reported improvements in cardiac outcomes present in these studies provide evidence for the paracrine hypothesis, and suggests that the factors released by MSCs could potentially be equally beneficial therapeutic options.
Anderson et al., took this premise a step further and trialled specific factors identified in vitro in a LAD model of I/R. They found that HGF, but not CXCL2, soaked micro-sponges could significantly reduce infarct size, improve cardiac function, and prevent CM apoptosis [21]. In line with these findings, Yeghiazarians et al. reported that delivery of bone marrow cell extract 3 days post MI, reduced infarct size and improved overall cardiac function and vessel density to a comparable extent to whole cell therapy [12]. A follow up from Yeghiazarians et al. demonstrated that IL-15, a factor identified as being highly expressed in the bone marrow cell extract, could protect CMs from cell death and oxidative stress under hypoxia in vitro [11]. Furthermore, they demonstrated that IL-15 can be protective in a model of mouse MI, by improving cardiac function, and reducing infarct size and CM cell death [106]. A study by Angeli et al., demonstrated that the administration of the cell extracts of human mononuclear cells and bone marrow cells 2 days post-MI in mice resulted in a significant increase in LVEF, vascular density at the border zone, and reduced infarct size [107]. In line with these findings, the data present in the included studies further demonstrate that the intact cell may not be essential to achieve cardiac repair.
In conclusion, this systematic review has enabled the identification and consolidation of 228 individual factors known to be secreted by MSCs, which may have protective effects in cardiac models of ischemia. In the included studies, a significant number investigated the effects of MSC therapy in vivo or ex vivo. Of particular interest were those that clearly demonstrated that treatment with either the conditioned media of MSCs or the factors identified within promote effects which are equally beneficial as whole cell therapy. Together these studies suggest that the release of soluble, pro-reparative factors by transplanted MSCs are responsible for the beneficial effects reported, providing strong support for the paracrine hypothesis of cardiac repair. The factors released by MSCs have significant potential to lead to the identification of novel therapeutic targets, thus making way for alternative and more effective therapeutic options for treating cardiac fibrosis and heart failure which could drastically improve the health outcomes of patients suffering from CVDs.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Avolio, E., et al. (2015). Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circulation Research, 116(10), e81-94.
Bao, L., et al. (2017). C-Kit Positive Cardiac Stem Cells and Bone Marrow-Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function After Myocardial Infarction in a Paracrine Manner. Journal of Cardiac Failure, 23(5), 403–415.
Huang, C., et al. (2011). Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS ONE, 6(12), e29246.
Assmus, B., et al. (2002). Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation, 106(24), 3009–3017.
Beltrami, A. P., et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell, 114(6), 763–776.
Messina, E., et al. (2004). Isolation and expansion of adult cardiac stem cells from human and murine heart. Circulation Research, 95(9), 911–921.
Nygren, J. M., et al. (2004). Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nature Medicine, 10(5), 494–501.
Deuse, T., et al. (2009). Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation, 120(11 Suppl), S247–S254.
Keith, M. C. L., et al. (2015). Safety of Intracoronary Infusion of 20 Million C-Kit Positive Human Cardiac Stem Cells in Pigs. PLoS ONE, 10(4), e0124227.
Angoulvant, D., et al. (2011). Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. Journal of Heart & Lung Transplantation, 30(1), 95–102.
Yeghiazarians, Y., et al. (2014). IL-15: A novel pro-survival signaling pathway in cardiomyocytes. Journal of Cardiovascular Pharmacology, 63(5), 406–411.
Yeghiazarians, Y., et al. (2009). Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Molecular Therapy, 17(7), 1250–1256.
See, F., et al. (2011). Therapeutic effects of human STRO-3-selected mesenchymal precursor cells and their soluble factors in experimental myocardial ischemia. Journal of Cellular & Molecular Medicine, 15(10), 2117–2129.
Gnecchi, M., et al. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circulation Research, 103(11), 1204–1219.
Danieli, P., et al. (2015). Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angiogenesis. Stem Cells Translational Medicine, 4(5), 448–458.
Kinnaird, T., et al. (2004). Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circulation Research, 94(5), 678–685.
Xu, M., et al. (2007). In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. Journal of Molecular and Cellular Cardiology, 42(2), 441–448.
Nikfarjam, S., et al. (2020). Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. Journal of Translational Medicine, 18(1), 449.
Yin, K., Wang, S., & Zhao, R. C. (2019). Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomarker Research, 7, 8–8.
Rosano, J. M., et al. (2012). Targeted Delivery of VEGF after a Myocardial Infarction Reduces Collagen Deposition and Improves Cardiac Function. Cardiovascular Engineering and Technology, 3(2), 237–247.
Anderson, C. D., et al. (2008). The role of cytoprotective cytokines in cardiac ischemia/reperfusion injury. Journal of Surgical Research, 148(2), 164–171.
Dominici, M., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317.
Bayes-Genis, A., et al. (2010). Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. Journal of Molecular & Cellular Cardiology, 49(5), 771–780.
Adutler-Lieber, S., et al. (2013). Human macrophage regulation via interaction with cardiac adipose tissue-derived mesenchymal stromal cells. Journal of Cardiovascular Pharmacology & Therapeutics, 18(1), 78–86.
Figeac, F., et al. (2014). Nanotubular crosstalk with distressed cardiomyocytes stimulates the paracrine repair function of mesenchymal stem cells. Stem Cells, 32(1), 216–230.
Li, Q., et al. (2021). CD73<sup>+</sup> Mesenchymal Stem Cells Ameliorate Myocardial Infarction by Promoting Angiogenesis. Frontiers in Cell & Developmental Biology, 9, 637239.
Sadat, S., et al. (2007). The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochemical & Biophysical Research Communications, 363(3), 674–679.
Yan, W., et al. (2020). N-Cadherin Overexpression Mobilizes the Protective Effects of Mesenchymal Stromal Cells Against Ischemic Heart Injury Through a beta-Catenin-Dependent Manner. Circulation Research, 126(7), 857–874.
Yang, J., et al. (2012). Human adipose tissue-derived stem cells protect impaired cardiomyocytes from hypoxia/reoxygenation injury through hypoxia-induced paracrine mechanism. Cell Biochemistry & Function, 30(6), 505–514.
Nakanishi, C., et al. (2011). Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circulation Journal, 75(9), 2260–2268.
Liu, M. L., et al. (2014). Anti-inflammatory peptides from cardiac progenitors ameliorate dysfunction after myocardial infarction. Journal of the American Heart Association, 3(6), e001101.
Montzka, K., et al. (2010). Growth factor and cytokine expression of human mesenchymal stromal cells is not altered in an in vitro model of tissue damage. Cytotherapy, 12(7), 870–880.
Abarbanell, A. M., et al. (2010). Toll-like receptor 2 mediates mesenchymal stem cell-associated myocardial recovery and VEGF production following acute ischemia-reperfusion injury. American Journal of Physiology - Heart & Circulatory Physiology, 298(5), H1529–H1536.
Alrefai, M. T., et al. (2019). Functional Assessment of Pluripotent and Mesenchymal Stem Cell Derived Secretome in Heart Disease. Annals of Stem Cell Research, 2(1), 29–36.
Augustin, M., et al. (2013). VEGF overexpression improves mesenchymal stem cell sheet transplantation therapy for acute myocardial infarction. Journal of Tissue Engineering and Regenerative Medicine, 7(9), 742–750.
Baffour, R., et al. (2006). Bone marrow-derived stem cell interactions with adult cardiomyocytes and skeletal myoblasts in vitro. Cardiovascular Revascularization Medicine, 7(4), 222–230.
Burlacu, A., et al. (2013). Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells & Development, 22(4), 643–653.
Cai, H., et al. (2019). Self-assembling peptide modified with QHREDGS as a novel delivery system for mesenchymal stem cell transplantation after myocardial infarction. FASEB Journal, fj201801768RR.
Chen, P., et al. (2014). Hypoxia preconditioned mesenchymal stem cells prevent cardiac fibroblast activation and collagen production via leptin. PLoS ONE, 9(8), e103587.
Crisostomo, P. R., et al. (2008). Embryonic stem cells attenuate myocardial dysfunction and inflammation after surgical global ischemia via paracrine actions. American Journal of Physiology - Heart & Circulatory Physiology, 295(4), H1726–H1735.
Crisostomo, P. R., et al. (2007). In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery, 142(2), 215–221.
Crisostomo, P. R., et al. (2007). Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: Role of the 55 kDa TNF receptor (TNFR1). Journal of Molecular & Cellular Cardiology, 42(1), 142–149.
Crisostomo, P. R., et al. (2006). High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock, 26(6), 575–580.
Dai, Y., et al. (2007). HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. Journal of Molecular & Cellular Cardiology, 42(6), 1036–1044.
Daltro, P. S., et al. (2017). Therapy with mesenchymal stromal cells or conditioned medium reverse cardiac alterations in a high-fat diet-induced obesity model. Cytotherapy, 19(10), 1176–1188.
Deng, B., et al. (2020). Nonadherent culture method promotes MSC-mediated vascularization in myocardial infarction via miR-519d/VEGFA pathway. Stem Cell Research and Therapy, 11(1) (no pagination).
Deuse, T., et al. (2010). HGF or VEGF gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Journal of Heart and Lung Transplantation, 1, S81.
Erwin, G. S., et al. (2009). Estradiol-treated mesenchymal stem cells improve myocardial recovery after ischemia. Journal of Surgical Research, 152(2), 319–324.
Fan, Y., et al. (2015). Local renin-angiotensin system regulates hypoxia-induced vascular endothelial growth factor synthesis in mesenchymal stem cells. International Journal of Clinical & Experimental Pathology, 8(3), 2505–2514.
Fan, L., et al. (2009). Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. European Journal of Heart Failure, 11(11), 1023–1030.
Huang, F., et al. (2013). Overexpression of miR-126 promotes the differentiation of mesenchymal stem cells toward endothelial cells via activation of PI3K/Akt and MAPK/ERK pathways and release of paracrine factors. Biological Chemistry, 394(9), 1223–1233.
Huang, F., et al. (2013). Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. International Journal of Molecular Medicine, 31(2), 484–492.
Ju, X., et al. (2018). Catalpol Promotes the Survival and VEGF Secretion of Bone Marrow-Derived Stem Cells and Their Role in Myocardial Repair After Myocardial Infarction in Rats. Cardiovascular Toxicology, 18(5), 471–481.
Li, J., et al. (2015). Transcriptional profiling reveals crosstalk between mesenchymal stem cells and endothelial cells promoting prevascularization by reciprocal mechanisms. Stem Cells & Development, 24(5), 610–623.
Li, Z.-H., et al. (2020). Rapamycin-Preactivated Autophagy Enhances Survival and Differentiation of Mesenchymal Stem Cells After Transplantation into Infarcted Myocardium. Stem Cell Reviews & Reports, 16(2), 344–356.
Li, H.-X., et al. (2020). Wnt11 preserves mitochondrial membrane potential and protects cardiomyocytes against hypoxia through paracrine signaling. Journal of Cellular Biochemistry, 121(2), 1144–1155.
Li, K.-S., et al. (2020). MiR-29a in mesenchymal stem cells inhibits FSTL1 secretion and promotes cardiac myocyte apoptosis in hypoxia-reoxygenation injury. Cardiovascular Pathology, 46, 107180.
Lin, M., et al. (2020). IGF-1 enhances BMSC viability, migration, and anti-apoptosis in myocardial infarction via secreted frizzled-related protein 2 pathway. Stem Cell Research and Therapy, 11(1) (no pagination).
Lu, W., et al. (2013). Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. International Journal of Cardiology, 165(2), 333–340.
Luo, Y., et al. (2012). Pretreating mesenchymal stem cells with interleukin-1beta and transforming growth factor-beta synergistically increases vascular endothelial growth factor production and improves mesenchymal stem cell-mediated myocardial protection after acute ischemia. Surgery, 151(3), 353–363.
Mao, Q., et al. (2019). ILK promotes survival and self-renewal of hypoxic MSCs via the activation of lncTCF7-Wnt pathway induced by IL-6/STAT3 signaling. Gene Therapy, 27, 27.
Markel, T. A., et al. (2008). VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. American Journal of Physiology - Heart & Circulatory Physiology, 295(6), H2308–H2314.
Meng, X., et al. (2018). Transplantation of mesenchymal stem cells overexpressing IL10 attenuates cardiac impairments in rats with myocardial infarction. Journal of Cellular Physiology, 233(1), 587–595.
Page, P., et al. (2014). Effect of serum and oxygen concentration on gene expression and secretion of paracrine factors by mesenchymal stem cells. International Journal of Cell Biology, 2014, 601063.
Paquet, J., et al. (2015). Oxygen tension regulates human mesenchymal stem cell paracrine functions. Stem Cells Translational Medicine, 4(7), 809–821.
Popescu, S., et al. (2021). Dual Stem Cell Therapy Improves the Myocardial Recovery Post-Infarction through Reciprocal Modulation of Cell Functions. International Journal of Molecular Sciences, 22(11), 26.
RajendranNair, D. S., Karunakaran, J., & Nair, R. R. (2017). Differential response of human cardiac stem cells and bone marrow mesenchymal stem cells to hypoxia-reoxygenation injury. Molecular & Cellular Biochemistry, 425(1–2), 139–153.
Sassoli, C., et al. (2011). Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: Clues for cardiac regeneration. Journal of Molecular & Cellular Cardiology, 51(3), 399–408.
Shan, S., et al. (2018). Growth arrest-specific gene 6 transfer promotes mesenchymal stem cell survival and cardiac repair under hypoxia and ischemia via enhanced autocrine signaling and paracrine action. Archives of Biochemistry & Biophysics, 660, 108–120.
Song, Y. S., et al. (2017). Bone marrow mesenchymal stem cell-derived vascular endothelial growth factor attenuates cardiac apoptosis via regulation of cardiac miRNA-23a and miRNA-92a in a rat model of myocardial infarction. PLoS ONE, 12(6), e0179972.
Song, S. W., et al. (2016). Proteomic Analysis and Identification of Paracrine Factors in Mesenchymal Stem Cell-Conditioned Media under Hypoxia. Cellular Physiology & Biochemistry, 40(1–2), 400–410.
Thej, C., et al. (2017). Development of a surrogate potency assay to determine the angiogenic activity of Stempeucel, a pooled, ex-vivo expanded, allogeneic human bone marrow mesenchymal stromal cell product. Stem Cell Research & Therapy, 8(1), 47.
Wairiuko, G. M., et al. (2007). Stem cells improve right ventricular functional recovery after acute pressure overload and ischemia reperfusion injury. Journal of Surgical Research, 141(2), 241–246.
Wang, D. G., et al. (2014). Cx43 in mesenchymal stem cells promotes angiogenesis of the infarcted heart independent of gap junctions. Molecular Medicine Reports, 9(4), 1095–1102.
Windmolders, S., et al. (2014). Mesenchymal stem cell secreted platelet derived growth factor exerts a pro-migratory effect on resident Cardiac Atrial appendage Stem Cells. Journal of Molecular & Cellular Cardiology, 66, 177–188.
Xia, W., et al. (2015). Macrophage migration inhibitory factor confers resistance to senescence through CD74-dependent AMPK-FOXO3a signaling in mesenchymal stem cells. Stem Cell Research & Therapy, 6, 82.
Yang, J., et al. (2021). Sevoflurane preconditioning promotes mesenchymal stem cells to relieve myocardial ischemia/reperfusion injury via TRPC6-induced angiogenesis. Stem Cell Research & Therapy, 12(1), 584.
Yu, X. Y., et al. (2009). The effects of mesenchymal stem cells on c-kit up-regulation and cell-cycle re-entry of neonatal cardiomyocytes are mediated by activation of insulin-like growth factor 1 receptor. Molecular & Cellular Biochemistry, 332(1–2), 25–32.
Zeng, B., et al. (2008). Paracrine action of HO-1-modified mesenchymal stem cells mediates cardiac protection and functional improvement. Cell Biology International, 32(10), 1256–1264.
Zhang, J., et al. (2015). Mesenchymal stem cells promote cardiac muscle repair via enhanced neovascularization. Cellular Physiology & Biochemistry, 35(3), 1219–1229.
Zhang, M., et al. (2007). SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB Journal, 21(12), 3197–3207.
Zhou, P., et al. (2021). Donor heart preservation with hypoxic-conditioned medium-derived from bone marrow mesenchymal stem cells improves cardiac function in a heart transplantation model. Stem Cell Research & Therapy, 12(1), 56.
Tang, J. M., et al. (2011). VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovascular Research, 91(3), 402–411.
Li, T. S., et al. (2012). Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. Journal of the American College of Cardiology, 59(10), 942–953.
Jiang, Z., et al. (2013). Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. Journal of Cellular & Molecular Medicine, 17(10), 1247–1260.
Duran, J. M., et al. (2013). Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circulation Research, 113(5), 539–552.
Constantinou, C., et al. (2020). Human pluripotent stem cell-derived cardiomyocytes as a target platform for paracrine protection by cardiac mesenchymal stromal cells. Scientific Reports, 10(1), 13016.
Crisostomo, V., et al. (2019). Dose-dependent improvement of cardiac function in a swine model of acute myocardial infarction after intracoronary administration of allogeneic heart-derived cells. Stem Cell Research & Therapy, 10(1), 152.
Cui, J., et al. (2016). Macrophage migration inhibitory factor promotes cardiac stem cell proliferation and endothelial differentiation through the activation of the PI3K/Akt/mTOR and AMPK pathways. International Journal of Molecular Medicine, 37(5), 1299–1309.
Fanton, Y., et al. (2016). Cardiac atrial appendage stem cells promote angiogenesis in vitro and in vivo. Journal of Molecular & Cellular Cardiology, 97, 235–244.
Latham, N., et al. (2013). Human blood and cardiac stem cells synergize to enhance cardiac repair when cotransplanted into ischemic myocardium. Circulation, 128(11 Suppl 1), S105–S112.
Li, C., et al. (2017). c-kit Positive Cardiac Outgrowth Cells Demonstrate Better Ability for Cardiac Recovery Against Ischemic Myopathy. Journal of Stem Cell Research & Therapy, 7(10).
McQuaig, R., et al. (2020). Combination of Cardiac Progenitor Cells From the Right Atrium and Left Ventricle Exhibits Synergistic Paracrine Effects In Vitro. Cell Transplantation, 29(no pagination).
Samal, R., et al. (2019). Global secretome analysis of resident cardiac progenitor cells from wild-type and transgenic heart failure mice: Why ambience matters. Journal of Cellular Physiology, 234(7), 10111–10122.
Zhao, Y., et al. (2020). GDF11 enhances therapeutic efficacy of mesenchymal stem cells for myocardial infarction via YME1L-mediated OPA1 processing. Stem Cells Translational Medicine, 9(10), 1257–1271.
Czapla, J., et al. (2016). Human Cardiac Mesenchymal Stromal Cells with CD105+CD34- Phenotype Enhance the Function of Post-Infarction Heart in Mice. PLoS ONE, 11(7), e0158745.
Iso, Y., et al. (2007). Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochemical & Biophysical Research Communications, 354(3), 700–706.
Bussche, L., & Van de Walle, G. R. (2014). Peripheral Blood-Derived Mesenchymal Stromal Cells Promote Angiogenesis via Paracrine Stimulation of Vascular Endothelial Growth Factor Secretion in the Equine Model. Stem Cells Translational Medicine, 3(12), 1514–1525.
Bader, A. M., et al. (2014). Mechanisms of paracrine cardioprotection by cord blood mesenchymal stromal cells. European Journal of Cardio-Thoracic Surgery, 45(6), 983–992.
Alijani-Ghazyani, Z., et al. (2021). Conditioned medium harvested from Hif1alpha engineered mesenchymal stem cells ameliorates LAD-occlusion -induced injury in rat acute myocardial ischemia model. International Journal of Biochemistry & Cell Biology, 130, 105897.
Takahashi, M., et al. (2006). Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. American Journal of Physiology. Heart and Circulatory Physiology, 291(2), H886–H893.
Nakamura, T., et al. (2000). Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. Journal of Clinical Investigation, 106.
Avolio, E., et al. (2014). Ex vivo molecular rejuvenation improves the therapeutic activity of senescent human cardiac stem cells in a mouse model of myocardial infarction. Stem Cells, 32(9), 2373–2385.
Toldo, S., et al. (2013). Interleukin-1β blockade improves cardiac remodelling after myocardial infarction without interrupting the inflammasome in the mouse. Experimental Physiology, 98(3), 734–745.
Ridker, P. M., et al. (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine, 377(12), 1119–1131.
Ameri, K., et al. (2020). Administration of Interleukin-15 Peptide Improves Cardiac Function in a Mouse Model of Myocardial Infarction. Journal of Cardiovascular Pharmacology, 75(1), 98–102.
Angeli, F. S., et al. (2012). Injection of human bone marrow and mononuclear cell extract into infarcted mouse hearts results in functional improvement. Open Cardiovascular Medicine Journal, 6, 38–43.
Acknowledgements
N.S.M. and S.A.H are supported by the Australian Commonwealth funded Research Training Program (RTP) stipend, they are PhD candidates in the program of Doctor of Philosophy (Medicine) at The University of Newcastle, Australia, and the Doctoral School for Translational Molecular and Cellular Biosciences at the Medical University of Graz, Austria. L.R. is a PhD candidate in Molecular Medicine at the Medical University of Graz, Austria.
This research was funded in whole, or in part, by the Austrian Science Fund (FWF) and ERA-CVD (AIR-MI, I 4168] to PPR. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
N.S.M., J.L., S.A.H., L.A.M., P.P.R., A.J.B. contributed to study concept and design. N.S.M., L.R., J.L., A.J.B. contributed to abstract and/ or full text screening, quality assessment and data acquisition. N.S.M. and L.R. analysed and interpreted data. N.S.M. drafted the manuscript. N.S.M., L.R., L.A.M., P.P.R., A.J.B. contributed to critical revision of the manuscript for intellectual content.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mabotuwana, N.S., Rech, L., Lim, J. et al. Paracrine Factors Released by Stem Cells of Mesenchymal Origin and their Effects in Cardiovascular Disease: A Systematic Review of Pre-clinical Studies. Stem Cell Rev and Rep 18, 2606–2628 (2022). https://doi.org/10.1007/s12015-022-10429-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10429-6