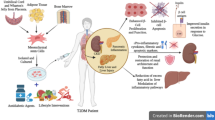
Abstract
Diabetes mellitus (DM), a chronic disorder of carbohydrate metabolism, is characterized by the unbridled hyperglycemia resulted from the impaired ability of the body to either produce or respond to insulin. As a cell-based regenerative therapy, mesenchymal stem cells (MSCs) hold immense potency for curing DM duo to their easy isolation, multi-differentiation potential, and immunomodulatory property. However, despite the promising efficacy in pre-clinical animal models, naive MSC administration fails to exhibit clinically satisfactory therapeutic outcomes, which varies greatly among individuals with DM. Recently, numbers of innovative strategies have been applied to improve MSC-based therapy. Preconditioning, genetic modification, combination therapy and exosome application are representative strategies to maximize the therapeutic benefits of MSCs. Therefore, in this review, we summarize recent advancements in mechanistic studies of MSCs-based treatment for DM, and mainly focus on the novel approaches aiming to improve the anti-diabetic potentials of naive MSCs. Additionally, the potential directions of MSCs-based therapy for DM are also proposed at a glance.
Graphical abstract



Similar content being viewed by others
Data Availability
The literatures in this study are available from the corresponding authors on reasonable request.
References
Kamal, M. M., & Kassem, D. H. (2020). Therapeutic potential of Wharton’s jelly mesenchymal stem cells for diabetes: Achievements and challenges. Frontiers in Cell and Developmental Biology, 8, 16.
Donzelli, E., & Scuteri, A. (2020). Mesenchymal stem cells: A trump card for the treatment of diabetes? Biomedicines, 8(5), 112.
Grohová, A., Dáňová, K., Špíšek, R., & Palová-Jelínková, L. (2019). Cell based therapy for type 1 diabetes: Should we take hyperglycemia into account? Frontiers in Immunology, 10, 79.
Qi, Y., Ma, J., Li, S., & Liu, W. (2019). Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Research & Therapy, 10(1), 274.
Keane, K. N., Calton, E. K., Carlessi, R., Hart, P. H., & Newsholme, P. (2017). The bioenergetics of inflammation: Insights into obesity and type 2 diabetes. European Journal of Clinical Nutrition, 71(7), 904–912.
Wang, M., Zhang, W., Xu, S., Peng, L., Wang, Z., Liu, H., Fang, Q., Deng, T., Men, X., & Lou, J. (2017). TRB3 mediates advanced glycation end product-induced apoptosis of pancreatic β-cells through the protein kinase C β pathway. International Journal of Molecular Medicine, 40(1), 130–136.
Samuel, V. T., & Shulman, G. I. (2016). The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. Journal of Clinical Investigation, 126(1), 12–22.
Nowak, C., Sundström, J., Gustafsson, S., Giedraitis, V., Lind, L., Ingelsson, E., & Fall, T. (2016). Protein biomarkers for insulin resistance and type 2 diabetes risk in two large community cohorts. Diabetes, 65(1), 276–284.
Zang, L., Hao, H., Liu, J., Li, Y., Han, W., & Mu, Y. (2017). Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetology & Metabolic Syndrome, 9, 36.
Sneddon, J. B., Tang, Q., Stock, P., Bluestone, J. A., Roy, S., Desai, T., & Hebrok, M. (2018). Stem cell therapies for treating diabetes: Progress and remaining challenges. Cell Stem Cell, 22(6), 810–823.
Forbes, J. M., & Cooper, M. E. (2013). Mechanisms of diabetic complications. Physiological Reviews, 93(1), 137–188.
Fradkin, J. E., & Rodgers, G. P. (2017). Glycemic therapy for type 2 diabetes: Choices expand, data lag behind. Annals of Internal Medicine, 166(4), 309–310.
Pathak, V., Pathak, N. M., O'Neill, C. L., Guduric-Fuchs, J., & Medina, R. J. (2019). Therapies for type 1 diabetes: Current scenario and future perspectives. Clinical Medicine Insights: Endocrinology and Diabetes, 12, 1179551419844521.
Chaudhury, A., Duvoor, C., Reddy Dendi, V. S., Kraleti, S., Chada, A., Ravilla, R., Marco, A., Shekhawat, N. S., Montales, M. T., Kuriakose, K., Sasapu, A., Beebe, A., Patil, N., Musham, C. K., Lohani, G. P., & Mirza, W. (2017). Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Frontiers in Endocrinology, 8, 6.
Sheykhhasan, M. (2019). Towards standardized stem cell therapy in type 2 diabetes mellitus: A systematic review. Current Stem Cell Research & Therapy, 14(1), 75–76.
Latres, E., Finan, D. A., Greenstein, J. L., Kowalski, A., & Kieffer, T. J. (2019). Navigating two roads to glucose normalization in diabetes: Automated insulin delivery devices and cell therapy. Cell Metabolism, 29(3), 545–563.
Kumar, S. A., Delgado, M., Mendez, V. E., & Joddar, B. (2019). Applications of stem cells and bioprinting for potential treatment of diabetes. World Journal of Stem Cells, 11(1), 13–32.
Zhang, Y., Chen, W., Feng, B., & Cao, H. (2020). The clinical efficacy and safety of stem cell therapy for diabetes mellitus: A systematic review and meta-analysis. Aging and Disease, 11(1), 141–153.
Kondo, Y., Toyoda, T., Inagaki, N., & Osafune, K. (2018). iPSC technology-based regenerative therapy for diabetes. Journal of Diabetes Investigation, 9(2), 234–243.
Päth, G., Perakakis, N., Mantzoros, C. S., & Seufert, J. (2019). Stem cells in the treatment of diabetes mellitus - focus on mesenchymal stem cells. Metabolism, 90, 1–15.
Velazco-Cruz, L., Song, J., Maxwell, K. G., Goedegebuure, M. M., Augsornworawat, P., Hogrebe, N. J., & Millman, J. R. (2019). Acquisition of Dynamic Function in human stem cell-derived β cells. Stem Cell Reports, 12(2), 351–365.
van der Torren, C. R., Zaldumbide, A., Duinkerken, G., Brand-Schaaf, S. H., Peakman, M., Stangé, G., Martinson, L., Kroon, E., Brandon, E. P., Pipeleers, D., & Roep, B. O. (2017). Immunogenicity of human embryonic stem cell-derived beta cells. Diabetologia, 60(1), 126–133.
Hrvatin, S., O'Donnell, C. W., Deng, F., Millman, J. R., Pagliuca, F. W., DiIorio, P., Rezania, A., Gifford, D. K., & Melton, D. A. (2014). Differentiated human stem cells resemble fetal, not adult, β cells. Proceedings of the National Academy of Sciences of the United States of America, 111(8), 3038–3043.
Sordi, V., Pellegrini, S., Krampera, M., Marchetti, P., Pessina, A., Ciardelli, G., Fadini, G., Pintus, C., Pantè, G., & Piemonti, L. (2017). Stem cells to restore insulin production and cure diabetes. Nutrition, Metabolism & Cardiovascular Diseases, 27(7), 583–600.
Couri, C. E., Oliveira, M. C., Stracieri, A. B., Moraes, D. A., Pieroni, F., Barros, G. M., Madeira, M. I., Malmegrim, K. C., Foss-Freitas, M. C., Simões, B. P., Martinez, E. Z., Foss, M. C., Burt, R. K., & Voltarelli, J. C. (2009). C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA, 301(15), 1573–1579.
D'Addio, F., Valderrama Vasquez, A., Ben Nasr, M., Franek, E., Zhu, D., Li, L., Ning, G., Snarski, E., & Fiorina, P. (2014). Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: A multicenter analysis. Diabetes, 63(9), 3041–3046.
Pittenger, M. F., Discher, D. E., Péault, B. M., Phinney, D. G., Hare, J. M., & Caplan, A. I. (2019). Mesenchymal stem cell perspective: cell biology to clinical progress. npj Regenerative Medicine, 4, 22.
Khaki, M., Salmanian, A. H., Abtahi, H., Ganji, A., & Mosayebi, G. (2018). Mesenchymal stem cells differentiate to endothelial cells using recombinant vascular endothelial growth factor -a. Reports of Biochemistry and Molecular Biology, 6(2), 144–150.
Ullah, I., Subbarao, R. B., & Rho, G. J. (2015). Human mesenchymal stem cells - current trends and future prospective. Bioscience Reports, 35(2), e00191.
Filho, D. M., de Carvalho Ribeiro, P., Oliveira, L. F., Dos Santos, A., Parreira, R. C., Pinto, M. C. X., & Resende, R. R. (2019). Enhancing the therapeutic potential of mesenchymal stem cells with the CRISPR-Cas system. Stem Cell Reviews and Reports, 15(4), 463–473.
Quevedo, H. C., Hatzistergos, K. E., Oskouei, B. N., Feigenbaum, G. S., Rodriguez, J. E., Valdes, D., Pattany, P. M., Zambrano, J. P., Hu, Q., McNiece, I., Heldman, A. W., & Hare, J. M. (2009). Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America, 106(33), 14022–14027.
Guo, X., Bai, Y., Zhang, L., Zhang, B., Zagidullin, N., Carvalho, K., Du, Z., & Cai, B. (2018). Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications. Stem Cell Research & Therapy, 9(1), 44.
Antonitsis, P., Ioannidou-Papagiannaki, E., Kaidoglou, A., Charokopos, N., Kalogeridis, A., Kouzi-Koliakou, K., Kyriakopoulou, I., Klonizakis, I., & Papakonstantinou, C. (2008). Cardiomyogenic potential of human adult bone marrow mesenchymal stem cells in vitro. Thoracic and Cardiovascular Surgeon, 56(2), 77–82.
Xu, W., Zhang, X., Qian, H., Zhu, W., Sun, X., Hu, J., Zhou, H., & Chen, Y. (2004). Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Experimental biology and medicine (Maywood, N.J.), 229(7), 623–631.
Rose, R. A., Jiang, H., Wang, X., Helke, S., Tsoporis, J. N., Gong, N., Keating, S. C., Parker, T. G., Backx, P. H., & Keating, A. (2008). Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells, 26(11), 2884–2892.
Liu, D. D., Ullah, M., Concepcion, W., Dahl, J. J., & Thakor, A. S. (2020). The role of ultrasound in enhancing mesenchymal stromal cell-based therapies. Stem Cells Translational Medicine, 9(8), 850–866.
Pires, A. O., Mendes-Pinheiro, B., Teixeira, F. G., Anjo, S. I., Ribeiro-Samy, S., Gomes, E. D., Serra, S. C., Silva, N. A., Manadas, B., Sousa, N., & Salgado, A. J. (2016). Unveiling the differences of Secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: A proteomic analysis. Stem Cells and Development, 25(14), 1073–1083.
Shi, Y., Wang, Y., Li, Q., Liu, K., Hou, J., Shao, C., & Wang, Y. (2018). Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nature Reviews Nephrology, 14(8), 493–507.
Wu, Y., Hoogduijn, M. J., Baan, C. C., Korevaar, S. S., de Kuiper, R., Yan, L., Wang, L., & van Besouw, N. M. (2017). Adipose tissue-derived mesenchymal stem cells have a heterogenic cytokine secretion profile. Stem Cells International, 2017, 4960831.
Xie, Z., Hao, H., Tong, C., Cheng, Y., Liu, J., Pang, Y., Si, Y., Guo, Y., Zang, L., Mu, Y., & Han, W. (2016). Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells, 34(3), 627–639.
Xie, M., Hao, H. J., Cheng, Y., Xie, Z. Y., Yin, Y. Q., Zhang, Q., Gao, J. Q., Liu, H. Y., Mu, Y. M., & Han, W. D. (2017). Adipose-derived mesenchymal stem cells ameliorate hyperglycemia through regulating hepatic glucose metabolism in type 2 diabetic rats. Biochemical and Biophysical Research Communications, 483(1), 435–441.
Yin, Y., Hao, H., Cheng, Y., Zang, L., Liu, J., Gao, J., Xue, J., Xie, Z., Zhang, Q., Han, W., & Mu, Y. (2018). Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell Death & Disease, 9(7), 760.
Lau, T. T., & Wang, D. A. (2011). Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opinion on Biological Therapy, 11(2), 189–197.
Yin, Y., Hao, H., Cheng, Y., Gao, J., Liu, J., Xie, Z., Zhang, Q., Zang, L., Han, W., & Mu, Y. (2018). The homing of human umbilical cord-derived mesenchymal stem cells and the subsequent modulation of macrophage polarization in type 2 diabetic mice. International Immunopharmacology, 60, 235–245.
Sood, V., Mittal, B. R., Bhansali, A., Singh, B., Khandelwal, N., Marwaha, N., & Jain, A. (2015). Biodistribution of 18F-FDG-labeled autologous bone marrow-derived stem cells in patients with type 2 diabetes mellitus: Exploring targeted and intravenous routes of delivery. Clinical Nuclear Medicine, 40(9), 697–700.
Ghoneim, M. A., Refaie, A. F., Elbassiouny, B. L., Gabr, M. M., & Zakaria, M. M. (2020). From mesenchymal stromal/stem cells to insulin-producing cells: Progress and challenges. Stem Cell Reviews and Reports, 16(6), 1156–1172.
Ghoneim, M. A., Refaie, A. F., Elbassiouny, B. L., Gabr, M. M., & Zakaria, M. M. (2020). From mesenchymal stromal/stem cells to insulin-producing cells: Progress and challenges. Stem Cell Research & Therapy, 16(6), 1156–1172.
El-Demerdash, R. F., Hammad, L. N., Kamal, M. M., & El Mesallamy, H. O. (2015). A comparison of Wharton's jelly and cord blood as a source of mesenchymal stem cells for diabetes cell therapy. Regenerative Medicine, 10(7), 841–855.
Wu, L. F., Wang, N. N., Liu, Y. S., & Wei, X. (2009). Differentiation of Wharton's jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Engineering Part A, 15(10), 2865–2873.
Tsai, P. J., Wang, H. S., Lin, G. J., Chou, S. C., Chu, T. H., Chuan, W. T., Lu, Y. J., Weng, Z. C., Su, C. H., Hsieh, P. S., Sytwu, H. K., Lin, C. H., Chen, T. H., & Shyu, J. F. (2015). Undifferentiated Wharton's jelly mesenchymal stem cell transplantation induces insulin-producing cell differentiation and suppression of T-cell-mediated autoimmunity in nonobese diabetic mice. Cell Transplantation, 24(8), 1555–1570.
Wang, M., Yuan, Q., & Xie, L. (2018). Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells International, 2018, 3057624.
Hu, J., Fu, Z., Chen, Y., Tang, N., Wang, L., Wang, F., Sun, R., & Yan, S. (2015). Effects of autologous adipose-derived stem cell infusion on type 2 diabetic rats. Endocrine Journal, 62(4), 339–352.
Zhao, K., Hao, H., Liu, J., Tong, C., Cheng, Y., Xie, Z., Zang, L., Mu, Y., & Han, W. (2015). Bone marrow-derived mesenchymal stem cells ameliorate chronic high glucose-induced β-cell injury through modulation of autophagy. Cell Death & Disease, 6(9), e1885.
Xu, Y., Tan, M., Ma, X., Li, H., He, X., Chen, Z., Tan, Y., Nie, W., Rong, P., & Wang, W. (2020). Human mesenchymal stem cells-derived conditioned medium inhibits hypoxia-induced death of neonatal porcine islets by inducing autophagy. Xenotransplantation, 27(1), e12556.
Hung, S. C., Pochampally, R. R., Chen, S. C., Hsu, S. C., & Prockop, D. J. (2007). Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells, 25(9), 2363–2370.
Mundra, V., Wu, H., & Mahato, R. I. (2013). Genetically modified human bone marrow derived mesenchymal stem cells for improving the outcome of human islet transplantation. PLoS One, 8(10), e77591.
Hu, J., Wang, F., Sun, R., Wang, Z., Yu, X., Wang, L., Gao, H., Zhao, W., Yan, S., & Wang, Y. (2014). Effect of combined therapy of human Wharton's jelly-derived mesenchymal stem cells from umbilical cord with sitagliptin in type 2 diabetic rats. Endocrine, 45(2), 279–287.
Som, C., & Venkataramana, N. K. (2018). Evaluation of efficacy and regenerative potential of Wharton’s jelly and bone marrow derived mesenchymal stem cells in diabetic rats. Journal of Pre-Clinical and Clinical Research., 12(1), 30–35.
Saeedi, P., Halabian, R., & Imani Fooladi, A. A. (2019). A revealing review of mesenchymal stem cells therapy, clinical perspectives and modification strategies. Stem Cell Investigation, 6, 34.
Aggarwal, S., & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105(4), 1815–1822.
Kim, D. W., Staples, M., Shinozuka, K., Pantcheva, P., Kang, S. D., & Borlongan, C. V. (2013). Wharton's jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. International Journal of Molecular Sciences, 14(6), 11692–11712.
Pirola, L., & Ferraz, J. C. (2017). Role of pro- and anti-inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World Journal of Biological Chemistry, 8(2), 120–128.
Sun, Y., Shi, H., Yin, S., Ji, C., Zhang, X., Zhang, B., Wu, P., Shi, Y., Mao, F., Yan, Y., Xu, W., & Qian, H. (2018). Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano, 12(8), 7613–7628.
Si, Y., Zhao, Y., Hao, H., Liu, J., Guo, Y., Mu, Y., Shen, J., Cheng, Y., Fu, X., & Han, W. (2012). Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: Identification of a novel role in improving insulin sensitivity. Diabetes, 61(6), 1616–1625.
Serena, C., Keiran, N., Ceperuelo-Mallafre, V., Ejarque, M., Fradera, R., Roche, K., Nuñez-Roa, C., Vendrell, J., & Fernández-Veledo, S. (2016). Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells, 34(10), 2559–2573.
Oñate, B., Vilahur, G., Camino-López, S., Díez-Caballero, A., Ballesta-López, C., Ybarra, J., Moscatiello, F., Herrero, J., & Badimon, L. (2013). Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genomics, 14, 625.
Chang, T. C., Hsu, M. F., & Wu, K. K. (2015). High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS One, 10(5), e0126537.
Sen, S., Domingues, C. C., Rouphael, C., Chou, C., Kim, C., & Yadava, N. (2015). Genetic modification of human mesenchymal stem cells helps to reduce adiposity and improve glucose tolerance in an obese diabetic mouse model. Stem Cell Research & Therapy, 6, 242.
Xu, J., Huang, Z., Lin, L., Fu, M., Song, Y., Shen, Y., Ren, D., Gao, Y., Su, Y., Zou, Y., Chen, Y., Zhang, D., Hu, W., Qian, J., & Ge, J. (2015). miRNA-130b is required for the ERK/FOXM1 pathway activation-mediated protective effects of isosorbide dinitrate against mesenchymal stem cell senescence induced by high glucose. International Journal of Molecular Medicine, 35(1), 59–71.
Seo, Y., Shin, T. H., & Kim, H. S. (2019). Current strategies to enhance adipose stem cell function: An update. International Journal of Molecular Sciences, 20(15), 3827.
Atashi, F., Modarressi, A., & Pepper, M. S. (2015). The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells and Development, 24(10), 1150–1163.
Chaudhari, P., Ye, Z., & Jang, Y. Y. (2014). Roles of reactive oxygen species in the fate of stem cells. Antioxidants & Redox Signaling, 20(12), 1881–1890.
Yang, S. R., Park, J. R., & Kang, K. S. (2015). Reactive oxygen species in mesenchymal stem cell aging: Implication to lung diseases. Oxidative Medicine and Cellular Longevity, 2015, 486263.
Ishizuka, T., Hinata, T., & Watanabe, Y. (2011). Superoxide induced by a high-glucose concentration attenuates production of angiogenic growth factors in hypoxic mouse mesenchymal stem cells. Journal of Endocrinology, 208(2), 147–159.
Zhang, H., Zhai, Z., Wang, Y., Zhang, J., Wu, H., Wang, Y., Li, C., Li, D., Lu, L., Wang, X., Chang, J., Hou, Q., Ju, Z., Zhou, D., & Meng, A. (2013). Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radical Biology and Medicine, 54, 40–50.
Chen, T. S., Kuo, C. H., Day, C. H., Pan, L. F., Chen, R. J., Chen, B. C., Padma, V. V., Lin, Y. M., & Huang, C. Y. (2019). Resveratrol increases stem cell function in the treatment of damaged pancreas. Journal of Cellular Physiology, 234(11), 20443–20452.
ShamsEldeen, A. M., Ashour, H., Shoukry, H. S., Fadel, M., Kamar, S. S., Aabdelbaset, M., Rashed, L. A., & Ammar, H. I. (2019). Combined treatment with systemic resveratrol and resveratrol preconditioned mesenchymal stem cells, maximizes antifibrotic action in diabetic cardiomyopathy. Journal of Cellular Physiology, 234(7), 10942–10963.
Goossens, G. H., & Blaak, E. E. (2012). Adipose tissue oxygen tension: Implications for chronic metabolic and inflammatory diseases. Current Opinion in Clinical Nutrition & Metabolic Care, 15(6), 539–546.
Tsai, C. C., Yew, T. L., Yang, D. C., Huang, W. H., & Hung, S. C. (2012). Benefits of hypoxic culture on bone marrow multipotent stromal cells. American Journal of Blood Research, 2(3), 148–159.
Hu, C., & Li, L. (2018). Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. Journal of Cellular and Molecular Medicine, 22(3), 1428–1442.
Ito, A., Aoyama, T., Yoshizawa, M., Nagai, M., Tajino, J., Yamaguchi, S., Iijima, H., Zhang, X., & Kuroki, H. (2015). The effects of short-term hypoxia on human mesenchymal stem cell proliferation, viability and p16(INK4A) mRNA expression: Investigation using a simple hypoxic culture system with a deoxidizing agent. Journal of Stem cells and Regenerative Medicine, 11(1), 25–31.
Chacko, S. M., Ahmed, S., Selvendiran, K., Kuppusamy, M. L., Khan, M., & Kuppusamy, P. (2010). Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. American Journal of Physiology-Cell Physiology, 299(6), C1562–C1570.
Estrada, J. C., Albo, C., Benguría, A., Dopazo, A., López-Romero, P., Carrera-Quintanar, L., Roche, E., Clemente, E. P., Enríquez, J. A., Bernad, A., & Samper, E. (2012). Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death & Differentiation, 19(5), 743–755.
Choi, J. R., Pingguan-Murphy, B., Wan Abas, W. A., Noor Azmi, M. A., Omar, S. Z., Chua, K. H., & Wan Safwani, W. K. (2014). Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochemical and Biophysical Research Communications, 448(2), 218–224.
Liu, L., Gao, J., Yuan, Y., Chang, Q., Liao, Y., & Lu, F. (2013). Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biology International, 37(6), 551–560.
Schive, S. W., Mirlashari, M. R., Hasvold, G., Wang, M., Josefsen, D., Gullestad, H. P., Korsgren, O., Foss, A., Kvalheim, G., & Scholz, H. (2017). Human adipose-derived mesenchymal stem cells respond to short-term hypoxia by secreting factors beneficial for human islets in vitro and potentiate antidiabetic effect in vivo. Cell Medicine, 9(3), 103–116.
Xiang, C., & Xie, Q. P. (2018). Protection of mouse pancreatic islet function by co-culture with hypoxia pre-treated mesenchymal stromal cells. Molecular Medicine Reports, 18(3), 2589–2598.
Najafi, R., & Sharifi, A. M. (2013). Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opinion on Biological Therapy, 13(7), 959–972.
Oses, C., Olivares, B., Ezquer, M., Acosta, C., Bosch, P., Donoso, M., Léniz, P., & Ezquer, F. (2017). Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS One, 12(5), e0178011.
Wei, W., Huang, Y., Li, D., Gou, H. F., & Wang, W. (2018). Improved therapeutic potential of MSCs by genetic modification. Gene Therapy, 25(8), 538–547.
Hodgkinson, C. P., Gomez, J. A., Mirotsou, M., & Dzau, V. J. (2010). Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Human Gene Therapy, 21(11), 1513–1526.
Banerjee, M., & Vats, P. (2014). Reactive metabolites and antioxidant gene polymorphisms in type 2 diabetes mellitus. Indian Journal of Human Genetics, 20(1), 10–19.
Baldari, S., Di Rocco, G., Trivisonno, A., Samengo, D., Pani, G., & Toietta, G. (2016). Promotion of survival and engraftment of transplanted adipose tissue-derived stromal and vascular cells by overexpression of manganese superoxide dismutase. International Journal of Molecular Sciences, 17(7), 1082.
Domingues, C. C., Kundu, N., Kropotova, Y., Ahmadi, N., & Sen, S. (2019). Antioxidant-upregulated mesenchymal stem cells reduce inflammation and improve fatty liver disease in diet-induced obesity. Stem Cell Research & Therapy, 10(1), 280.
Röder, P. V., Wu, B., Liu, Y., & Han, W. (2016). Pancreatic regulation of glucose homeostasis. Experimental and Molecular Medicine, 48(3), e219.
Gagliardino, J. J. (2005). Physiological endocrine control of energy homeostasis and postprandial blood glucose levels. European Review for Medical and Pharmacological Sciences, 9(2), 75–92.
Hui, H., Farilla, L., Merkel, P., & Perfetti, R. (2002). The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. European Journal of Endocrinology, 146(6), 863–869.
Gao, L. R., Zhang, N. K., Zhang, Y., Chen, Y., Wang, L., Zhu, Y., & Tang, H. H. (2018). Overexpression of apelin in Wharton' jelly mesenchymal stem cell reverses insulin resistance and promotes pancreatic β cell proliferation in type 2 diabetic rats. Stem Cell Research & Therapy, 9(1), 339.
Trahair, L. G., Horowitz, M., Stevens, J. E., Feinle-Bisset, C., Standfield, S., Piscitelli, D., Rayner, C. K., Deane, A. M., & Jones, K. L. (2015). Effects of exogenous glucagon-like peptide-1 on blood pressure, heart rate, gastric emptying, mesenteric blood flow and glycaemic responses to oral glucose in older individuals with normal glucose tolerance or type 2 diabetes. Diabetologia, 58(8), 1769–1778.
Grunnet, L. G., & Mandrup-Poulsen, T. (2011). Cytokines and type 1 diabetes: A numbers game. Diabetes, 60(3), 697–699.
Nauck, M. A. (2004). Glucagon-like peptide 1 (GLP-1) in the treatment of diabetes. Hormone and Metabolic Research, 36(11–12), 852–858.
Chang, Y., Dong, M., Wang, Y., Yu, H., Sun, C., Jiang, X., Chen, W., Wang, X., Xu, N., Liu, W., & Jin, N. (2019). GLP-1 gene-modified human umbilical cord mesenchymal stem cell line improves blood glucose level in type 2 diabetic mice. Stem Cells International, 2019, 4961865.
Yi, P., Park, J. S., & Melton, D. A. (2013). Betatrophin: A hormone that controls pancreatic β cell proliferation. Cell, 153(4), 747–758.
Sun, L. L., Liu, T. J., Li, L., Tang, W., Zou, J. J., Chen, X. F., Zheng, J. Y., Jiang, B. G., & Shi, Y. Q. (2017). Transplantation of betatrophin-expressing adipose-derived mesenchymal stem cells induces β-cell proliferation in diabetic mice. International Journal of Molecular Medicine, 39(4), 936–948.
Yue, P., Jin, H., Aillaud, M., Deng, A. C., Azuma, J., Asagami, T., Kundu, R. K., Reaven, G. M., Quertermous, T., & Tsao, P. S. (2010). Apelin is necessary for the maintenance of insulin sensitivity. American Journal of Physiology-Endocrinology and Metabolism, 298(1), E59–E67.
Antushevich, H., & Wójcik, M. (2018). Review: Apelin in disease. Clinica Chimica Acta, 483, 241–248.
Fournel, A., Drougard, A., Duparc, T., Marlin, A., Brierley, S. M., Castro, J., Le-Gonidec, S., Masri, B., Colom, A., Lucas, A., Rousset, P., Cenac, N., Vergnolle, N., Valet, P., Cani, P. D., & Knauf, C. (2017). Apelin targets gut contraction to control glucose metabolism via the brain. Gut, 66(2), 258–269.
Bertrand, C., Valet, P., & Castan-Laurell, I. (2015). Apelin and energy metabolism. Frontiers in Physiology, 6, 115.
Chen, H., Zheng, C., Zhang, X., Li, J., Li, J., Zheng, L., & Huang, K. (2011). Apelin alleviates diabetes-associated endoplasmic reticulum stress in the pancreas of Akita mice. Peptides, 32(8), 1634–1639.
Dray, C., Knauf, C., Daviaud, D., Waget, A., Boucher, J., Buléon, M., Cani, P. D., Attané, C., Guigné, C., Carpéné, C., Burcelin, R., Castan-Laurell, I., & Valet, P. (2008). Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metabolism, 8(5), 437–445.
Thowfeequ, S., Ralphs, K. L., Yu, W. Y., Slack, J. M., & Tosh, D. (2007). Betacellulin inhibits amylase and glucagon production and promotes beta cell differentiation in mouse embryonic pancreas. Diabetologia, 50(8), 1688–1697.
Nakano, Y., Furuta, H., Doi, A., Matsuno, S., Nakagawa, T., Shimomura, H., Sakagashira, S., Horikawa, Y., Nishi, M., Sasaki, H., Sanke, T., & Nanjo, K. (2005). A functional variant in the human betacellulin gene promoter is associated with type 2 diabetes. Diabetes, 54(12), 3560–3566.
Silver, K., Tolea, M., Wang, J., Pollin, T. I., Yao, F., & Mitchell, B. D. (2005). The exon 1 Cys7Gly polymorphism within the betacellulin gene is associated with type 2 diabetes in African Americans. Diabetes, 54(4), 1179–1184.
Oh, Y. S., Shin, S., Lee, Y. J., Kim, E. H., & Jun, H. S. (2011). Betacellulin-induced beta cell proliferation and regeneration is mediated by activation of ErbB-1 and ErbB-2 receptors. PLoS One, 6(8), e23894.
Oh, Y. S., Shin, S., Li, H. Y., Park, E. Y., Lee, S. M., Choi, C. S., Lim, Y., Jung, H. S., & Jun, H. S. (2015). Betacellulin ameliorates hyperglycemia in obese diabetic db/db mice. Journal of Molecular Medicine, 93(11), 1235–1245.
Shin, S., Li, N., Kobayashi, N., Yoon, J. W., & Jun, H. S. (2008). Remission of diabetes by β-cell regeneration in diabetic mice treated with a recombinant adenovirus expressing Betacellulin. Molecular Therapy, 16(5), 854–861.
Paz, A. H., Salton, G. D., Ayala-Lugo, A., Gomes, C., Terraciano, P., Scalco, R., Laurino, C. C., Passos, E. P., Schneider, M. R., Meurer, L., & Cirne-Lima, E. (2011). Betacellulin overexpression in mesenchymal stem cells induces insulin secretion in vitro and ameliorates streptozotocin-induced hyperglycemia in rats. Stem Cells and Development, 20(2), 223–232.
Sauter, N. S., Schulthess, F. T., Galasso, R., Castellani, L. W., & Maedler, K. (2008). The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology, 149(5), 2208–2218.
Volarevic, V., Al-Qahtani, A., Arsenijevic, N., Pajovic, S., & Lukic, M. L. (2010). Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity, 43(4), 255–263.
Stosić-Grujicić, S., Lukić, M., & Ostajić, N. (1994). [interleukin 1 receptor antagonists prevent the induction of experimental insulin-dependent autoimmune diabetes]. Srpski Arhiv Za Celokupno Lekarstvo, 122 Suppl 1, 11-12.
Larsen, C. M., Faulenbach, M., Vaag, A., Vølund, A., Ehses, J. A., Seifert, B., Mandrup-Poulsen, T., & Donath, M. Y. (2007). Interleukin-1-receptor antagonist in type 2 diabetes mellitus. New England Journal of Medicine, 356(15), 1517–1526.
An, T., Chen, Y., Tu, Y., & Lin, P. (2020). Mesenchymal stromal cell-derived extracellular vesicles in the treatment of diabetic foot Ulcers: Application and Challenges. Stem Cell Reviews and Reports. doi: https://doi.org/10.1007/s12015-020-10014-9.
Jia, Y., Cao, N., Zhai, J., Zeng, Q., Zheng, P., Su, R., Liao, T., Liu, J., Pei, H., Fan, Z., Zhou, J., Xi, J., He, L., Chen, L., Nan, X., Yue, W., & Pei, X. (2020). HGF mediates clinical-grade human umbilical cord-derived mesenchymal stem cells improved functional recovery in a senescence-accelerated mouse model of Alzheimer's disease. Advanced Science, 7(17), 1903809.
Suzuki, J., Shimamura, M., Suda, H., Wakayama, K., Kumagai, H., Ikeda, Y., Akazawa, H., Isobe, M., Komuro, I., & Morishita, R. (2016). Current therapies and investigational drugs for peripheral arterial disease. Hypertension Research, 39(4), 183–191.
Jing, Y., Sun, Q., Xiong, X., Meng, R., Tang, S., Cao, S., Bi, Y., & Zhu, D. (2019). Hepatocyte growth factor alleviates hepatic insulin resistance and lipid accumulation in high-fat diet-fed mice. Journal of Diabetes Investigation, 10(2), 251–260.
Araújo, T. G., Oliveira, A. G., Carvalho, B. M., Guadagnini, D., Protzek, A. O., Carvalheira, J. B., Boschero, A. C., & Saad, M. J. (2012). Hepatocyte growth factor plays a key role in insulin resistance-associated compensatory mechanisms. Endocrinology, 153(12), 5760–5769.
Wu, H., Lu, W., & Mahato, R. I. (2011). Mesenchymal stem cells as a gene delivery vehicle for successful islet transplantation. Pharmaceutical Research, 28(9), 2098–2109.
Lee, H. S., Lee, J. G., Yeom, H. J., Chung, Y. S., Kang, B., Hurh, S., Cho, B., Park, H., Hwang, J. I., Park, J. B., Ahn, C., Kim, S. J., & Yang, J. (2016). The introduction of human Heme Oxygenase-1 and soluble tumor necrosis factor-α receptor type I with human IgG1 fc in porcine islets prolongs islet xenograft survival in humanized mice. American Journal of Transplantation, 16(1), 44–57.
Öllinger, R., & Pratschke, J. (2010). Role of heme oxygenase-1 in transplantation. Transplant International, 23(11), 1071–1081.
Machen, J., Bertera, S., Chang, Y., Bottino, R., Balamurugan, A. N., Robbins, P. D., Trucco, M., & Giannoukakis, N. (2004). Prolongation of islet allograft survival following ex vivo transduction with adenovirus encoding a soluble type 1 TNF receptor-Ig fusion decoy. Gene Therapy, 11(20), 1506–1514.
Lee, H. S., Song, S., Shin, D. Y., Kim, G. S., Lee, J. H., Cho, C. W., Lee, K. W., Park, H., Ahn, C., Yang, J., Yang, H. M., Park, J. B., & Kim, S. J. (2018). Enhanced effect of human mesenchymal stem cells expressing human TNF-αR-fc and HO-1 gene on porcine islet xenotransplantation in humanized mice. Xenotransplantation, 25(1). https://doi.org/10.1111/xen.12342.
Gao, J., Cheng, Y., Hao, H., Yin, Y., Xue, J., Zhang, Q., Li, L., Liu, J., Xie, Z., Yu, S., Li, B., Han, W., & Mu, Y. (2019). Decitabine assists umbilical cord-derived mesenchymal stem cells in improving glucose homeostasis by modulating macrophage polarization in type 2 diabetic mice. Stem Cell Research & Therapy, 10(1), 259.
Issa, J. P., Gharibyan, V., Cortes, J., Jelinek, J., Morris, G., Verstovsek, S., Talpaz, M., Garcia-Manero, G., & Kantarjian, H. M. (2005). Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. Journal of Clinical Oncology, 23(17), 3948–3956.
Xue, J., Cheng, Y., Hao, H., Gao, J., Yin, Y., Yu, S., Zou, J., Liu, J., Zhang, Q., & Mu, Y. (2020). Low-dose Decitabine assists human umbilical cord-derived mesenchymal stem cells in protecting β cells via the modulation of the macrophage phenotype in type 2 diabetic mice. Stem Cells International, 2020, 4689798.
Katzeff, H. L., Williams-Herman, D., Xu, L., Golm, G. T., Wang, H., Dong, Q., Johnson, J. R., O'Neill, E. A., Kaufman, K. D., Engel, S. S., & Goldstein, B. J. (2015). Long-term efficacy of sitagliptin as either monotherapy or add-on therapy to metformin: Improvement in glycemic control over 2 years in patients with type 2 diabetes. Current Medical Research and Opinion, 31(6), 1071–1077.
Monami, M., Iacomelli, I., Marchionni, N., & Mannucci, E. (2010). Dipeptydil peptidase-4 inhibitors in type 2 diabetes: A meta-analysis of randomized clinical trials. Nutrition, Metabolism & Cardiovascular Diseases, 20(4), 224–235.
Zhang, Q., Xiao, X., Zheng, J., & Li, M. (2019). A glucagon-like peptide-1 analog, liraglutide, ameliorates endothelial dysfunction through miRNAs to inhibit apoptosis in rats. PeerJ, 7, e6567.
Li, L. R., Jia, X. L., Hui, H., Zhang, J., Liu, Y., Cui, W. J., Xu, Q. Y., & Zhu, D. L. (2016). Liraglutide enhances the efficacy of human mesenchymal stem cells in preserving islet β-cell function in severe non-obese diabetic mice. Molecular Medicine, 22, 800–808.
Wang, W., Wu, R. D., Chen, P., Xu, X. J., Shi, X. Z., Huang, L. H., Shao, Z. L., & Guo, W. (2020). Liraglutide combined with human umbilical cord mesenchymal stem cell transplantation inhibits beta-cell apoptosis via mediating the ASK1/JNK/BAX pathway in rats with type 2 diabetes. Diabetes-Metabolism Research and Review, 36(2), e3212.
Chen, P., Huang, Q., Xu, X. J., Shao, Z. L., Huang, L. H., Yang, X. Z., Guo, W., Li, C. M., & Chen, C. (2016). The effect of liraglutide in combination with human umbilical cord mesenchymal stem cells treatment on glucose metabolism and β cell function in type 2 diabetes mellitus. Zhonghua Nei Ke Za Zhi, 55(5), 349–354.
Dadheech, N., Srivastava, A., Vakani, M., Shrimali, P., Bhonde, R., & Gupta, S. (2020). Direct lineage tracing reveals Activin-a potential for improved pancreatic homing of bone marrow mesenchymal stem cells and efficient ß-cell regeneration in vivo. Stem Cell Research & Therapy, 11(1), 327.
Xia, Y., & Schneyer, A. L. (2009). The biology of activin: Recent advances in structure, regulation and function. Journal of Endocrinology, 202(1), 1–12.
Zhang, Y. Q., Cleary, M. M., Si, Y., Liu, G., Eto, Y., Kritzik, M., Dabernat, S., Kayali, A. G., & Sarvetnick, N. (2004). Inhibition of activin signaling induces pancreatic epithelial cell expansion and diminishes terminal differentiation of pancreatic beta-cells. Diabetes, 53(8), 2024–2033.
Gomzikova, M. O., James, V., & Rizvanov, A. A. (2019). Therapeutic application of mesenchymal stem cells derived extracellular vesicles for immunomodulation. Frontiers in Immunology, 10, 2663.
Keerthikumar, S., Chisanga, D., Ariyaratne, D., Al Saffar, H., Anand, S., Zhao, K., Samuel, M., Pathan, M., Jois, M., Chilamkurti, N., Gangoda, L., & Mathivanan, S. (2016). ExoCarta: A web-based compendium of Exosomal cargo. Journal of Molecular Biology, 428(4), 688–692.
Phinney, D. G., & Pittenger, M. F. (2017). Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells, 35(4), 851–858.
Miceli, V., Pampalone, M., Vella, S., Carreca, A. P., Amico, G., & Conaldi, P. G. (2019). Comparison of immunosuppressive and Angiogenic properties of human amnion-derived mesenchymal stem cells between 2D and 3D culture systems. Stem Cells International, 2019, 7486279.
Casado-Díaz, A., Quesada-Gómez, J. M., & Dorado, G. (2020). Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: Applications in skin wound healing. Frontiers in Bioengineering and Biotechnology, 8, 146.
He, Q., Wang, L., Zhao, R., Yan, F., Sha, S., Cui, C., Song, J., Hu, H., Guo, X., Yang, M., Cui, Y., Sun, Y., Sun, Z., Liu, F., Dong, M., Hou, X., & Chen, L. (2020). Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Research & Therapy, 11(1), 223.
Sabry, D., Marzouk, S., Zakaria, R., Ibrahim, H. A., & Samir, M. (2020). The effect of exosomes derived from mesenchymal stem cells in the treatment of induced type 1 diabetes mellitus in rats. Biotechnology Letters, 42(8), 1597–1610.
Nojehdehi, S., Soudi, S., Hesampour, A., Rasouli, S., Soleimani, M., & Hashemi, S. M. (2018). Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. Journal of Cellular Biochemistry, 119(11), 9433–9443.
Lamichhane, T. N., Sokic, S., Schardt, J. S., Raiker, R. S., Lin, J. W., & Jay, S. M. (2015). Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Engineering Part B: Reviews, 21(1), 45–54.
Ding, J., Wang, X., Chen, B., Zhang, J., & Xu, J. (2019). Exosomes derived from human bone marrow mesenchymal stem cells stimulated by Deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. BioMed Research International, 2019, 9742765.
Liu, W., Yu, M., Xie, D., Wang, L., Ye, C., Zhu, Q., Liu, F., & Yang, L. (2020). Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Research & Therapy, 11(1), 259.
Yu, M., Liu, W., Li, J., Lu, J., Lu, H., Jia, W., & Liu, F. (2020). Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Research & Therapy, 11(1), 350.
Wang, B., Yao, K., Huuskes, B. M., Shen, H. H., Zhuang, J., Godson, C., Brennan, E. P., Wilkinson-Berka, J. L., Wise, A. F., & Ricardo, S. D. (2016). Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Molecular Therapy, 24(7), 1290–1301.
Tao, S. C., Guo, S. C., Li, M., Ke, Q. F., Guo, Y. P., & Zhang, C. Q. (2017). Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Translational Medicine, 6(3), 736–747.
Ngoc, P. K., Phuc, P. V., Nhung, T. H., Thuy, D. T., & Nguyet, N. T. (2011). Improving the efficacy of type 1 diabetes therapy by transplantation of immunoisolated insulin-producing cells. Human Cell, 24(2), 86–95.
El-Halawani, S. M., Gabr, M. M., El-Far, M., Zakaria, M. M., Khater, S. M., Refaie, A. F., & Ghoneim, M. A. (2020). Subcutaneous transplantation of bone marrow derived stem cells in macroencapsulation device for treating diabetic rats; clinically transplantable site. Heliyon, 6(5), e03914.
Sabek, O. M., Farina, M., Fraga, D. W., Afshar, S., Ballerini, A., Filgueira, C. S., Thekkedath, U. R., Grattoni, A., & Gaber, A. O. (2016). Three-dimensional printed polymeric system to encapsulate human mesenchymal stem cells differentiated into islet-like insulin-producing aggregates for diabetes treatment. Journal of Tissue Engineering, 7, 2041731416638198.
Barati, G., Nadri, S., Hajian, R., Rahmani, A., Mostafavi, H., Mortazavi, Y., & Taromchi, A. H. (2019). Differentiation of microfluidic-encapsulated trabecular meshwork mesenchymal stem cells into insulin producing cells and their impact on diabetic rats. Journal of Cellular Physiology, 234(5), 6801–6809.
Gabr, M. M., Zakaria, M. M., Refaie, A. F., Ismail, A. M., Khater, S. M., Ashamallah, S. A., Azzam, M. M., & Ghoneim, M. A. (2018). Insulin-producing cells from adult human bone marrow mesenchymal stromal cells could control chemically induced diabetes in dogs: A preliminary study. Cell Transplantation, 27(6), 937–947.
Teotia, R. S., Kadam, S., Singh, A. K., Verma, S. K., Bahulekar, A., Kanetkar, S., & Bellare, J. (2017). Islet encapsulated implantable composite hollow fiber membrane based device: A bioartificial pancreas. Materials Science & Engineering C-Materials for Biological Applications, 77, 857–866.
Kadam, S. S., Sudhakar, M., Nair, P. D., & Bhonde, R. R. (2010). Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy, 12(8), 982–991.
Acknowledgments
This work was supported by grants from National Key Research and Development Program of China (Grant No. 2017YFA0103902 & 2019YFA0111400), the National Natural Science Foundation of China (Grant No. 31771283), Innovative Research Team of High-level Local Universities in Shanghai (Grant No. SSMU-ZDCX20180700) and a Key Laboratory Program of the Education Commission of Shanghai Municipality (Grant No. ZDSYS14005).
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
HS.L. performed the literature search, devised the conceptual ideas, and wrote the manuscript. H.Z. performed the literature search, and wrote the manuscript. T.G. performed the literature search. ZF.W. devised the conceptual ideas, wrote the manuscript, and critically revised the manuscript. C.Z. initiated the study, revised the manuscript, and supervised the project.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable. This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent to Participate
Informed consents have been acquired from all participants.
Consent for Publication
All participants approve this article to be published.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Zhu, H., Ge, T. et al. Mesenchymal Stem Cell-Based Therapy for Diabetes Mellitus: Enhancement Strategies and Future Perspectives. Stem Cell Rev and Rep 17, 1552–1569 (2021). https://doi.org/10.1007/s12015-021-10139-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10139-5