Abstract
Ulcerative colitis (UC) is an idiopathic inflammatory disease. We intend to explore the mechanism of Rutin in the therapy of UC. Disease activity index (DAI) and hematoxylin-eosin staining were employed to assess therapeutic effect of Rutin on dextran sulfate sodium-stimulated mice. The proliferation was detected by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide assay. Oxidative stress (OS) was assessed by measuring reactive oxygen species (ROS), malondialdehyde (MDA), and superoxide dismutase (SOD). Inflammatory factors were detected using enzyme-linked immunosorbent assay and immunofluorescence staining. mRNA and protein expressions were detected by real-time quantitative polymerase chain reaction and immunoblotting assay. Rutin decreased DAI scores and ameliorated pathological damage in UC mice with decreased levels of inflammatory factors. Rutin recovered the inhibited proliferation of fetal human colon cells caused by lipopolysaccharide. Rutin inhibited OS by reducing ROS and MDA, while enhancing SOD activity in LPS-induced fetal human colon cells. Rutin inhibited NLRP3 inflammasome in UC mice and cell model. Silencing NLRP3 enhanced the inhibitory effect of Rutin on OS in lipopolysaccharide-induced fetal human colon cells. Conversely, NLRP3 overexpression reversed the restraining role of Rutin in OS. Rutin ameliorates UC by inhibiting inflammation and OS through suppressing NLRP3 inflammasome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a chronic, idiopathic inflammatory disease that destroys the colon, which primarily affects people aged 30–40 years and causes impairment of colon [1]. Mucosal inflammation extending from the rectum to the colon is the primary feature of UC [2]. UC lesions typically cause superficial damage to the intestinal wall, resulting in bloody diarrhea; due to its increasing occurrence in both industrialized and developing nations, as well as the significant increase in colorectal cancer morbidity and mortality that it causes, UC is gradually becoming a global burden [3, 4]. Currently, the main treatments for UC include 5-aminosalicylic acid, glucocorticoids, immunosuppressive medications, and colectomy, but issues including drug resistance development, patients’ intolerance to postoperative pouch anastomotic leakage, stomach infection, and intestinal obstruction are encountered [5, 6]. Therefore, searching for novel and effective therapies for UC is urgent.
The NLR family pyrin domain-containing 3 (NLRP3) inflammasomes are generally expressed in neutrophils, macrophages, lymphocytes, etc., which are composed of the Apoptosis-Associated Speck-Like Protein (ASC) and the pro-caspase-1 [7]. NLRP3 inflammasome assembly triggers the release of proinflammatory cytokines, Interleukin-1(IL-1) β and IL-18, which are caspase-1 dependent [8]. The activation of NLRP3 inflammasome is related to various molecular patterns of damage, including hyperglycemia, hypercalcemia, hypokalemia, etc. [9]. Abnormal activation of NLRP3 inflammatory vesicles can trigger an overactive immunological response, activating caspase-1, pro-IL-18, and pro-IL-1β and releasing pro-inflammatory cytokines, leading to the onset and progression of inflammatory bowel disease [10]. Additionally, the NLRP3 inflammasome is a crucial modulator of host defense that also maintains the normal morphology of intestinal epithelial cells to the microbiome and intestinal homeostasis [11]. However, the exact effect of the NLRP3 inflammasome on UC is largely unknown.
Oxidative stress (OS) is induced by an abnormal generation of reactive oxygen species (ROS) and antioxidants in defense system [12]. ROS are naturally occurring by products of cellular aerobic metabolism [13]. ROS is vital in normal body homeostasis, which can maintain an optimal level in the cellular environment [14]. OS occurs when ROS is overproduced [15]. OS is an initiating factor in various diseases, such as cardiovascular disease, neurological disease, chronic lung and renal disease, and inflammatory bowel disease [16, 17]. Malondialdehyde (MDA) is a direct indicator of lipid peroxidation level in vivo, indicating OS and the generation of ROS. Superoxide dismutase (SOD) is an important member of antioxidant enzymes, which can remove superoxide free radicals and is an important molecule for maintaining cell homeostasis [18,19,20]. Studies have reported that a high oxidant payload causes increased generation of ROS, inflammation, transmembrane permeability, and DNA damage, thus leading to UC [21,22,23]. Nevertheless, the specific mechanism of OS in UC needs to be further elucidated.
Rutin, commonly known as rutoside or vitamin P, is a citrus flavonoid glycoside composed of the flavonol quercetin and the disaccharide rutinose [24, 25]. The biological processes and pharmacological actions of Rutin include detoxifying, lipid-lowering, antioxidant, antibacterial, anti-inflammatory, antiapoptotic, vasodilation, and anticancer properties [26, 27]. Therefore, Rutin is commonly applied as an antioxidant, anticancer, anti-inflammatory, antidiabetic, and antihypertensive agent [28]. Previous studies have shown that Rutin can reduce lipopolysaccharide (LPS)-induced muscle cell inflammation and human bronchial epithelial cell line apoptosis by inhibiting OS [29, 30]. Nevertheless, the effect of Rutin on UC remains unknown and needs to be further determined.
In the present research, we focused on the therapeutic effect of Rutin on UC mice. Subsequently, we conducted an in vitro experiment using LPS-induced fetal human colon cells (FHCs) to elucidate the regulatory mechanism of Rutin in the therapy of UC. Our research aims to provide a scientific rationale for Rutin in the treatment of UC. Furthermore, we hope to elucidate the underlying mechanism of the role of Rutin in UC and the therapeutic application of it.
Materials and Methods
DSS-induced UC Mouse Model
Sixty C57BL/6J male mice (specific pathogen-free grade), weighing 20.2 g ± 2 g were acquired from SPF Biotechnology Co., Ltd. (Beijing, China) and housed with 10 mice per cage. The rearing environment was as follows: specific pathogen, central air conditioning continuous ventilation, 22–26 °C room temperature, 50–65% relative humidity, and 12-h automatic light and dark cycle. All animal perdures were performed in compliance with the guidelines of laboratory animal care and approved by the Ethics Committee of The Affiliated Hospital, Southwest Medical University.
The mice in this study were grouped as follows: the normal control group (NC), DSS group, high dose of Rutin group (RH), middle dose of Rutin group (RM), low dose of Rutin group (RL), and mesalazine positive control group (MSLZ group), with 10 mice per group. The UC model was induced by oral administration of DSS in drinking water according to the previous reports [31]. DSS (3%) was orally administered to mice of DSS group, RH group, RM group, RL group, and MSLZ group in drinking water for 7 consecutive days. In the RH, RM, and RL groups, animals were administrated 100 mg/kg/d, 50 mg/kg/d, and 25 mg/kg/d of Rutin (dissolved in 1% sodium carboxymethyl cellulose) by gavage on the first day of modeling. The MSLZ group was treated with MSLZ sustained-release granules (Ethypharm, Shanghai, China) by gavage according to the following dosing: mice dosage (g / kg) = human daily oral dosage (g) × 0.0026 / 0.02 kg. The NC group and DSS group were given an equal volume of l% sodium carboxymethyl cellulose solution by gavage. Body weight, stool characteristics, and blood in the feces were observed and noted daily to calculate the disease activity index (DAI). After modeling, the mice were killed by cervical vertebrae. The colons were partially preserved at −80 °C and partially fixed using paraformaldehyde. The blood was collected for subsequent experiments.
Hematoxylin-Eosin (HE) Staining and Histological Index (HI)
Pathological changes of colon tissues of UC mice were assessed by HE staining. The tissues were dried, embedded, and sliced into 5 μm sections. After dewaxing with xylene and rehydrating with ethanol, hematoxylin (H9627, Sigma-Aldrich, St. Louis, MO, USA) was used for 5 min, and 0.5% eosin (E4009, Sigma-Aldrich) for 1 min. The pathological alterations were observed under a microscope. Three random fields were selected from each HE-stained slide for scoring. The specific scoring rules were as follows: 0 represented normal morphology and no inflammation, 1 represented goblet cell loss and mild infiltration, 2 represented a large goblet cell loss area and moderate infiltration, 3 represented crypt loss and extensive inflammatory infiltration of the mucosal muscular layer with mucosal edema and thickening, and 4 represented a large area of crypt loss and extensive inflammatory infiltration of the submucosa layer [32]. Scoring was performed following double-blind principle, and the average value was calculated for statistical analysis.
Cell Culture and Treatment
The FHCs (China Center for Type Culture Collection, Wuhan, China) were cultured in Dulbecco’s modified Eagle’s medium-F12 (DMEM; Gibco, Waltham, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Sigma‑Aldrich) in a humid incubator with 5% CO2 at 37 °C. Cells were grouped as follows: normal control (NC) group, LPS group (treatment with 1 μg/mL, 5 μg/mL, and 10 μg/mL LPS), and Rutin group (treatment with 12.5 μmol/L, 25 μmol/L, and 50 umol/L of Rutin), and N-acetylcysteine (NAC) group. FHCs cells were stimulated with LPS (Sigma-Aldrich) for 24 h to establish a cellular inflammatory model. Cells were treated with 10 mM NAC, which was applied as an inhibitor of ROS. Silencing of NLRP3 in FHCs was conducted by transfecting with small interfering RNA (siRNA; GeneChem Biotechnology Co. Ltd, Shanghai, China). The siNLRP3 target sequence was 5′-CACGCTAATGATCGACTTCAA-3′ (Qiagen). The overexpression of NLRP3 in FHCs was performed by transfecting with lentivirus vectors (GeneChem Biotechnology Co. Ltd). Transfection was carried out following the instructions of the Lipofectamine 3000 liposome transfection kit (Invitrogen, Carlsbad, CA, USA) [33].
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Assay
FHCs (logarithmic growth phase) were cultured in 96-well plates at the rate of 1× 104/ well. Cells were treated with LPS and Rutin at different concentrations and cultured for 6, 12, and 24 h, respectively. Each group was set up with 5 compound wells and cell-free wells (containing only pure medium), each well was added with 20 μL MTT regent (2 mg/mL), and the culture was continued for 4 h. Subsequently, dimethyl sulfoxide solution (150 μL) was added in each well. After shaking for 10 min, the optical density at 570 nm of each well was noted by a microplate reader.
Detection of ROS
ROS was detected using the DCFH-DA probe in the ROS detection kit (Beyotime, Shanghai, China). In brief, cells were incubated in DMEM-F12 culture medium without serum and antibiotics. Then DCFH-DA probe was added into cells and incubated for 30 min. The fluorescence values were recorded by a microplate reader, and the probe was observed by a fluorescence microscope (Leica, Weztlar, Germany).
Detection of MDA and SOD
The cell supernatant was collected and the MDA and SOD were detected using commercial kits (Beyotime) according to the instructions. Optical density at 532 nm and 450 nm were recorded by a microplate reader, respectively.
Immunofluorescence Staining
FHCs were fixed in 4% paraformaldehyde and permeabilized by 0.5% Triton X-100 for 15 min. Cells were blocked with 10% goat serum in phosphate-buffered saline at room temperature for 1 h and cultured with IL-1β antibody (1:400, abs120224, Absin, Shanghai, China) and IL-18 (1:400, ab243091, Abcam, Cambridge, MA, USA) antibody in 5% bovine serum albumin solution overnight at 4 °C. Cells were subsequently cultured with fluorescence goat anti-rabbit secondary antibody (1:200; Abcam) for 2 h. Finally, the nuclei were stained blue using 4, 6-diamidino-2-phenylindole (Molecular Probes; Thermo Fisher Scientific, Waltham, USA). Images were collected with a fluorescent microscope. Three to five fields were randomly screened out for each slide, and differences in fluorescence expression were analyzed using Image pro-plus 6.0 software.
Real-time Quantitative Polymerase Chain Reaction (RT‒qPCR)
RNA isolation of colon tissues and FHCs cells was performed with TRIZOL reagent (Beyotime). The concentration and purity of RNA were quantified using a Nano Drop Nano100 spectrophotometer (Thermo Fisher Scientific) and reverse transcribed into cDNA using Super Script IV Cells Direct cDNA synthesis kit (Thermo Fisher Scientific). The cDNA synthesis was conducted using the PCR amplification apparatus with the following reaction procedures: 25 °C for 5 min; 42 °C for 30 min; 85 °C for 5 s. The RT-qPCR experiment was performed using the ABI7500 quantitative PCR apparatus (Applied Biosystems, Foster City, CA, USA). The specific procedures were as follows: pre-denatured at 95 °C for 30 s, denatured at 95 °C for 10 s, annealed at 60 °C for 30 s, and 40 cycles. The Ct values were analyzed by the 2-ΔΔCt method. Each assay was performed three times. All primer sequences were listed as below: NLRP3: F: 5′-ATGGCTGTGTGGATCTTTGC-3′, R: 5′-CACGTGTCATTCCACTCTGG-3′; IL-1β: F: 5’-GCGGCCAGGATATAACTGACTTC-3’, R: 5′-TCCACATTCAGCACAGGACTCTC-3′; ASC: F: 5′-TTGCTGGATGCTCTGTATGG-3′, R: 5′-CAGGTCTGTCACCAAGTAGG-3′; Caspase-1: F: 5′- GCACAAGACCTCTGACAGCA-3′, R: 5′- TTGGGCAGTTCTTGGTATTC-3′; β-actin: F: 5′- CCTGACTGACTACCTCATGAAG-3′, R: 5′-GACGTGGAGAGCTTCTCCTTA-3′.
Western Blot
The Lysis buffer was employed to extract protein from colon tissues and FHCs cells (Beyotime). Protein concentrations were determined using bicinchoninic acid kit (Sigma-Aldrich). The protein samples were denatured at 95 °C for 5 min. The samples were separated on the 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were then transferred to the polyvinylidene fluoride membranes. After being blocked with 5% skim milk, the blots were then incubated with the following primary antibodies: anti-NLRP3 (1:1000, ab263899, Abcam);anti-cleaved caspase-1 (1:2000, Y-P80622, MedChemExpressNew Jersey, USA); anti-cleaved IL-1β (1:2000, abs120224, Absin); anti-IL-18 (1:1000, ab243091, Abcam), and anti-β-actin antibody (1:1000, ab179467, Abcam) at 4 °C overnight. Afterwards, proteins were incubated with the horseradish peroxidase-labeled secondary antibody (1:2000, ab288151, Abcam) for 1 h. The protein was developed using a gel imager (Bio-Rad, California, USA). The relative gray value of protein expression was obtained by dividing the gray value of the protein band by the gray value of the internal reference β-actin.
Enzyme-linked Immune Sorbent Assay (ELISA)
The colon tissues were put into a tissue homogenizer. After grinding, the supernatant of colon tissue homogenate was obtained by centrifuge at 12,000 × g for 20 min. The serum samples were extracted from the blood by centrifuge. The levels of IL-1β, IL-18, TNF-α and IL-6 in serum, colon homogenate, and cell supernatant were determined by ELISA kits (Esebio Biotechnology Co., Shanghai, China) following the instructions. The absorbance at 450 nm was detected using a microplate reader.
Statistical Analysis
The statistical analysis was carried out using SPSS 19.0, and the data were represented as mean ± standard deviation. T-tests were carried out for comparisons between two groups, while ANOVA analysis and Tukey’s multiple comparisons test were employed for comparisons between multiple groups. A statistically significant difference was presented as a P < 0.05.
Results
Rutin Ameliorates UC in Mice Model
The mice in the DSS group showed lethargy, disheveled fur, and mental depression on the second day of modeling. Among them, two mice had positive fecal occult blood. After three days of modeling, the mice of DSS group exhibited obvious clinical symptoms: severe diarrhea, occult blood in the stool or bloody stools, and loss of weight. The mice in RH group, RM group, and MSLZ group also developed clinical manifestations related to UC, but their symptoms were milder, compared with the DSS group. The DAI of the DSS group was elevated, compared to the NC group (P < 0.05), while the RH group, RM group, and MSLZ group had lower DAI, relative to the DSS group (P < 0.05). There was no significant difference in DAI between the RH group and MSLZ group (P > 0.05) (Fig. 1A).
Rutin treatment decreases the disease activity index (DAI) and ameliorates pathological damages in ulcerative colitis (UC) mice. A DAI scores in different groups. B Hematoxylin & eosin staining of colonic tissue (Amplification: 100×, Scale: 100 μm). C HI scores in different groups. D Body weight changes in different groups. E Colon length in different groups. *P < 0.05 vs. NC group, #P < 0.05 vs. DSS group, &P < 0.05 vs. RL group
In the UC model group, the colonic mucosa of mice presented obvious congestion and edema, with erosion, severe erosion, and multiple round or oval ulcers. The edges of the ulcers were congested and edematous, with infiltration of inflammatory cells. The HI scores of the colon in the DSS group were considerably higher than those of the NC group (P < 0.05), indicating that DSS successfully induced inflammation and ulceration of the mucosa in UC model. The HI scores in the RH group, RM group, and MSLZ group were decreased, relative to the UC model group (P < 0.05) (Fig. 1B, C). In addition, DSS intervention led to a decrease in the body weight of mice and a shortening of the colon length (P < 0.05). The body weight loss rate and colon length of the RL, RM, RH, and MSLZ groups recovered significantly compared with the DSS group (P < 0.05). In addition, the body weight loss rate of the RH and MSLZ groups was lower than that of the RL group, while the colon length was longer than that of the RL group (P < 0.05) (Fig. 1D, E).
Rutin Decreases IL-1β and IL-18 in UC Mice
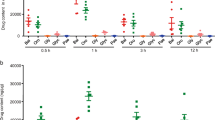
The ELISA results demonstrated that mice in DSS group had higher levels of IL-1β, IL-18, TNF-α and IL-6 in the serum and colon tissue, compared to the NC group (P < 0.05). Mice in the RH, RM, RL, and MSLZ groups had significantly lower levels of IL-1β, IL-18, TNF-α and IL-6 in the serum and colon tissue, compared to the DSS group (P < 0.05). Additionally, the RH group had lower IL-1β, IL-18, TNF-α and IL-6 levels than those in the MSLZ group (P < 0.05) (Fig. 2).
Rutin Inhibits the NLRP3 Inflammasome in UC Mice
The mRNA expression levels of NLRP3 and IL-1β in the DSS group were upregulated compared to the NC group (P < 0.05). Rutin and MSLZ treatment decreased the mRNA expression levels of NLRP3 and IL-1β in colon tissue of mice in the RH, RM, RL, and MSLZ groups. The mRNA expression levels of NLRP3 and IL-1β were considerably downregulated in the RM, RH, and MSLZ groups, relative to the DSS group (P < 0.05). The RH group showed lower mRNA expression levels of NLRP3 and IL-1β than that in the MSLZ group (P < 0.05) (Fig. 3A).
Rutin inhibits the NLRP3 inflammasome in UC mice. A The mRNA expression levels of NLRP3 and IL-1β were detected by real-time quantitative polymerase chain reaction (RT-qPCR). B The expression of NLRP3, cleaved Caspase-1, and cleaved IL-1β proteins in the colon tissue was detected by western blot. *P < 0.05 vs. NC group, #P < 0.05 vs. DSS group, &P < 0.05 vs. RL group
Mice in the DSS group had notably higher levels of NLRP3, cleaved Caspase-1, and cleaved IL-1β proteins than those in the NC group (P < 0.05). Furthermore, compared to the DSS group, the expression levels of NLRP3, cleaved Caspase-1, and cleaved IL-1β proteins in the colon tissue of mice in the RM group, RH group, and MSLZ group were downregulated (P < 0.05). Additionally, the levels of NLRP3, cleaved Caspase-1, and cleaved IL-1β in the RH group were considerably lower, relative to the MSLZ group (P < 0.05) (Fig. 3B).
Rutin Promotes the Proliferation of LPS-induced FHCs
The MTT results suggested that LPS inhibited cell proliferation and showed significant differences in the inhibitory effects of 1 μg/mL, 5 μg/mL, and 10 μg/mL of LPS on cell proliferation at 6 h, 12 h, and 24 h, which showed a time-dose dependent manner. Therefore, we selected 10 μg/ml LPS for 24 h (P < 0.05) as the modeling condition of FHCs inflammation model in vitro (Fig. 4A).
The proliferation was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. A The effect of lipopolysaccharide (LPS) on proliferation of fetal human colon cells (FHCs) was assessed by MTT assay. B The effect of Rutin on proliferation of FHCs was detected by MTT assay. C: Rutin reversed the inhibitory effect of LPS on cell proliferation in a time-concentration dependent manner. *P < 0.05 vs. NC group, #P < 0.05 vs. LPS group
MTT results illustrated that Rutin (12.5–50 μmol/L) had no significant impact on the proliferation of FHCs cells (P > 0.05), and the corresponding solvent DMSO matrix of 50 μmol/L in Rutin groups also had no significant impact on the proliferation of FHCs cells (P > 0.05). Therefore, we choose 12.5 μmol/L, 25 μmol/L, and 50 umol/L of Rutin as intervention reagents (Fig. 4B).
Compared with the NC group, LPS (10 μg/mL) significantly suppressed the proliferation of FHCs cells (P < 0.01). The treatment of Rutin (12.5–50 μmol/L) reversed the cell proliferation inhibited by LPS in a time-dose dependent manner (P < 0.05). The dose of 50 μmol/L Rutin showed no significant effect on the proliferation of FHCs (Fig. 4C).
Rutin Inhibits OS in LPS-induced FHCs
The ROS and MDA levels in LPS-induced FHCs were higher than those in the NC group (P < 0.01). After Rutin and NAC intervention, the generation of ROS and MDA was markedly inhibited (P < 0.01) and showed a concentration-dependent effect (Fig. 5A, B).
Relative to the NC group, the SOD level in FHCs cell of the LPS group was significantly reduced (P < 0.05). Rutin intervention significantly increased the concentrations of SOD in a concentration-dependent manner (P < 0.05). There was no significant difference in SOD levels between the NAC intervention group and Rutin intervention group (P > 0.05) (Fig. 5C).
Rutin Inhibits the NLRP3 Inflammasome in LPS-induced FHCs
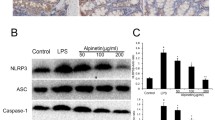
The western blot results demonstrated that compared to the NC group, the expression of NLRP3, ASC, cleaved caspase-1, and cleaved IL-1β markedly elevated after LPS stimulation (P < 0.05). Compared to the LPS group, the levels of NLRP3, ASC, cleaved caspase-1, and cleaved IL-1β were significantly decreased in the Rutin group and NAC group (P < 0.05) (Fig. 6A). LPS markedly enhanced the fluorescence intensities of IL-1β and IL-18 (P < 0.05), compared to the NC group. The expression of IL-1β and IL-18 proteins was significantly downregulated after Rutin or NAC intervention (P < 0.05) (Fig. 6B).
Rutin inhibits the NLRP3 inflammasome in LPS-induced FHCs. A The expression of NLRP3, Apoptosis-associated Speck-like protein (ASC), cleaved Caspase-1, and cleaved IL-1β proteins was detected by western blot. B The immunofluorescence staining of inflammatory factors, IL-1 β and IL-18 (Amplification: 200×, Scale: 100 μm). C The mRNA expression levels of NLRP3, ASC, Caspase-1, and IL-1β were detected by RT-qPCR. *P < 0.05 vs. NC group, #P < 0.05 vs. LPS group
The mRNA expression levels of NLRP3, ASC, caspase-1, and IL-1β were significantly higher than those in NC group after the stimulation of LPS (P < 0.05). Compared with the LPS group, the mRNA expression levels of NLRP3, ASC, caspase-1, and IL-1β were significantly reduced after the intervention of Rutin and NAC (P < 0.05) (Fig. 6C).
Rutin Inhibits OS in LPS-induced FHCs via Suppressing NLRP3 Inflammasome
Compared with the LV-CON235 group, the expression of NLRP3 in LV-NLRP3 group was considerably upregulated (Fig. 7A, B). Furthermore, the expression of NLRP3 was significantly decreased after transfecting with small interfering RNA (Fig. 7C, D). According to the transfection results, siRNA-NLRP3-2 target sequence 5′-CACGCTAATGATCGACTTCAA-3′ was used in subsequent experiments.
Compared to the Rutin group, the ROS and MDA levels were significantly decreased, while the levels of SOD were increased after NLRP3 silencing (Fig. 8A–C). Furthermore, the mRNA expression levels of ASC, caspase-1, and IL-1β in si-NLRP3 group were lower, relative to the Rutin group (Fig. 8D, E). Additionally, the expression of ASC, cleaved caspase-1, and cleaved IL-1β proteins was significantly inhibited in the si-NLRP3 group, compared to the Rutin group (Fig. 8F, G). Compared to the Rutin group, the concentrations of IL-1β and IL-18 were significantly reduced after NLRP3 silencing (Fig. 8H). Conversely, overexpression of NLRP3 caused an increase in the levels of ROS and MDA, while a decrease in the levels of SOD, compared to the Rutin group (Fig. 8A–C). The mRNA expression levels of ASC, caspase-1, and IL-1β in si-NLRP3 were apparently higher, compared to the Rutin group (Fig. 8D, E). Additionally, the expression of ASC, cleaved caspase-1, and cleaved IL-1β was considerably elevated after NLRP3 overexpression, compared to the Rutin group (Fig. 8F–I). Additionally, overexpression of NLRP3 caused an increase in the levels of IL-1β, IL-18, TNF-α and IL-6, compared to the Rutin group (Fig. 8J, K).
Rutin inhibits oxidative stress in LPS-induced FHCs via suppressing NLRP3 inflammasome signaling. A The generation of ROS. B The concentration of MDA. C The activity of SOD. D The mRNA expression levels of Caspase-1 and IL-1β were detected by RT-qPCR. E The mRNA expression levels of NLRP3 and ASC were detected by RT-qPCR. F, G The expression of cleaved Caspase-1 and cleaved IL-1β proteins was detected by western blot. H, I The expression of NLRP3 and ASC was detected by western blot. J, K The concentrations of inflammatory factors, IL-1β, IL-18, TNF-α and IL-6 were detected by enzyme-linked immunosorbent assay. *P < 0.05 vs. NC group, #P < 0.05 vs. LPS group
Discussion
Currently, a plethora of evidence supports the vital role of traditional Chinese medicine in treating UC: “platycodon,” “scutellaria,” and “honeysuckle,” have significant potential in the treatment of UC [34]. Herein, we found that Rutin significantly decreased DAI, HI scores, IL-1β, and IL-18 in UC model mice, and reduced the levels of NLRP3, caspase-1, and IL-1β in colon tissue. In vitro experiments demonstrated that Rutin had no significant effect on proliferation but could reverse the inhibitory effect of LPS on proliferation of FHCs. Rutin inhibited the generation of ROS and MDA induced by LPS in FHCs, reversed the inhibited SOD induced by LPS, and blocked the NLRP3 inflammasome signaling in LPS-induced FHCs cells. Overexpression of NLRP3 reversed the restraining role of Rutin in ROS and MDA, while suppressing SOD with increased levels of IL-1β and IL-18 in LPS-induced FHCs cells.
In this investigation, Rutin alleviated the DSS-stimulated UC in mice with decreased levels of inflammatory factors. In vitro, Rutin could reverse the inhibitory impact on proliferation and decrease the expression of IL-1β and IL-18 in LPS-induced FHCs. Studies have shown that traditional Chinese medicine can suppress the occurrence and development of UC by playing anti-inflammatory, antioxidant, and regulating immune response [34]. Sinapic acid can reduce the clinical symptoms and pathological changes of chronic colitis mice, as well as reduce the inflammatory factors [35]. Hypericum sampsonii Hance significantly alleviates LPS-induced inflammation by maintaining the unbalanced levels of inflammatory factors [36]. The haungqing (Scutellariae Radix) and baishao (Paeoniae Radix Alba) herb pairs can reduce the DAI scores and colon length, as well as the expression of TNF-α, IL-6, and IL-1β in UC mice [37]. Taken together, Rutin attenuates UC by exerting anti-inflammatory effects.
Research has pointed out that patients with inflammatory bowel diseases have enhanced OS and weakened antioxidant protection [38, 39]. In this research, the ROS and MDA were increased, while the SOD activity was inhibited in DSS-induced UC mice and LPS-induced FHCs. Notably, Rutin alleviated OS, as reflected by decreased levels of ROS and MDA and enhanced activity of SOD. The study has indicated that the antioxidant effect of Rutin is achieved by enhancing antioxidant enzymes, activating Nrf2/HO-1 pathway, and reducing MDA [40]. Many compounds have shown beneficial effects on OS and related diseases. Numerous substances found in carica papaya exhibit strong antioxidant qualities, such as Rutin, which can neutralize pro-oxidants by activating an antioxidant defense mechanism that inhibits OS through multiple signaling pathways, thereby promoting the expression of antioxidant enzymes or reducing the production of ROS [41]. Therefore, Rutin acts as an antioxidant in UC mice and LPS-induced FHCs, thus alleviating UC.
NLRP3 inflammasome: NLRP3 receptors, ASC connectors, and caspase-1 effectors can cleat IL-1β precursors to IL-1β, thereby exacerbating inflammation [42, 43]. The activation of NLRP3 inflammasome has been proven to aggravate DSS-induced colitis in mice [44, 45]. In this research, the NLRP3 inflammasome was activated in colon tissue of DSS-induced mice and LPS-induced FHCs. Rutin can suppress the NLRP3 inflammasome. Additionally, overexpression of NLRP3 could counteract the inhibitory role of Rutin in the OS in LPS-stimulated FHCs. By suppressing the production of NLRP3 inflammations (NLRP3, ASC, cleaved-caspase 1), betaine prevents colonic inflammatory cell pyroptosis and relieves acute severe UC by preventing OS-induced pyroptosis [46]. Apigenin plays an anti-inflammatory role by inhibiting the typical and atypical NLRP3 inflammasome pathway, regulating the cleavage of caspase-1 and caspase-11 enzymes, and reducing the expression of IL-1β and IL-18 [47, 48]. Due to the antioxidant and anti-inflammatory activities of phytochemicals, these compounds may be applied to treat diseases related to OS and inflammation [49]. Walnut oil can improve the pathological morphology of UC, reduce the production of ROS and the levels of pro-inflammatory cytokines, inhibit the NLRP3/ASC/Caspase-1 inflammatory pathway, and thus play an anti-inflammatory role in UC [50]. All in all, Rutin exerts anti-inflammatory and antioxidant effects in UC mice and LPS-stimulated FHCs by inhibiting NLRP3 inflammasome signaling.
In conclusion, this research demonstrates that Rutin is a secure and effective treatment for UC management. Rutin exhibited anti-inflammatory and antioxidant properties in UC mice and LPS-induced FHCs. Moreover, the inhibitory impact of Rutin on the OS was counteracted in vitro by overexpressing NLRP3. Mechanically, the application of Rutin attenuates the inflammation and OS of UC via suppressing NLRP3 inflammasome. Taken together, our findings lay the theoretical foundation for the application of Rutin in the treatment of UC and provide promising targets for the treatment and intervention of UC.
Data Availability
All data can be obtained by contacting the corresponding author.
References
Høivik, M. L., et al. (2013). Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut, 62(3), 368–375.
Ungaro, R., et al. (2017). Ulcerative colitis. Lancet, 389(10080), 1756–1770.
Kobayashi, T., et al. (2020). Ulcerative colitis. Nature Reviews Disease Primers, 6(1), 74.
Olén, O., et al. (2020). Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet, 395(10218), 123–131.
Gaetano, G., Gustavo, K. P., & Antonino, S. (2018). Surgery in ulcerative colitis: When? How?. Best Practice & Research Clinical Gastroenterology, 32–33, 71–78.
Le, Q., et al. (2013). Does a history of postoperative ileus predispose to recurrent ileus after multistage ileal pouch-anal anastomosis? Techniques in Coloproctology, 17(4), 383–388.
Liao, L. Z., et al. (2021). NLRP3 inflammasome activation contributes to the pathogenesis of cardiocytes aging. Aging (Albany NY), 13(16), 20534–20551.
Swanson, K. V., Deng, M., & Ting, J. P. (2019). The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews Immunology, 19(8), 477–489.
Xue, J. C., et al. (2023). Natural products modulate NLRP3 in ulcerative colitis. Frontiers in Pharmacology, 14, 1265825.
Zhang, J., et al. (2023). NLRP3: A Promising Therapeutic Target for Inflammatory Bowel Disease. Current Drug Targets, 24(14), 1106–1116.
Li, P., et al. (2022). Live Lactobacillus acidophilus alleviates ulcerative colitis via the SCFAs/mitophagy/NLRP3 inflammasome axis. Food and Function, 13(5), 2985–2997.
Marrocco, I., Altieri, F., & Peluso, I. (2017). Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Medicine and Cellular Longevity, 2017, 6501046.
Mailloux, R. J. (2020). An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants (Basel), 9(6), 472.
Singh, A., et al. (2019). Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. International Journal of Molecular Sciences, 20(17), 4258.
Heid, M. E., et al. (2013). Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. Journal of Immunology, 191(10), 5230–5238.
Ahmed, S. M., et al. (2017). Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et Biophysica Acta: Molecular Basis of Disease, 1863(2), 585–597.
Costantino, S., Paneni, F., & Cosentino, F. (2016). Ageing, metabolism and cardiovascular disease. The Journal of Physiology, 594(8), 2061–2073.
Kucharzik, T., et al. (2001). Neutrophil Transmigration in Inflammatory Bowel Disease Is Associated with Differential Expression of Epithelial Intercellular Junction Proteins. The American Journal of Pathology, 159(6), 2001–2009.
Förstermann, U., Xia, N., & Li, H. (2017). Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circulation Research, 120(4), 713–735.
Koutroubakis, I. E., et al. (2004). Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Digestive Diseases and Sciences, 49(9), 1433–1437.
Juan, C. A., et al. (2021). The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. International Journal of Molecular Sciences, 22(9), 4642.
Sies, H., & Jones, D. P. (2020). Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature Reviews Molecular Cell Biology, 21(7), 363–383.
Nakase, H., et al. (2022). The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmunity Reviews, 21(3), 103017.
Muvhulawa, N., et al. (2022). Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacological Research, 178, 106163.
Khajevand-Khazaei, M. R., et al. (2018). Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. European Journal of Pharmacology, 833, 307–313.
Bispo da Silva, A., et al. (2017). The flavonoid rutin modulates microglial/macrophage activation to a CD150/CD206 M2 phenotype. Chemico-Biological Interactions, 274, 89–99.
Yeh, C. H., et al. (2014). Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free Radical Biology and Medicine, 69, 249–257.
Hertog, M. G., et al. (1993). Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutrition and Cancer, 20(1), 21–29.
Liu, S., et al. (2019). Rutin attenuates inflammatory responses induced by lipopolysaccharide in an in vitro mouse muscle cell (C2C12) model. Poultry Science, 98(7), 2756–2764.
Paudel, K. R., et al. (2020). Rutin loaded liquid crystalline nanoparticles inhibit lipopolysaccharide induced oxidative stress and apoptosis in bronchial epithelial cells in vitro. Toxicology in Vitro, 68, 104961.
Wirtz, S., et al. (2017). Chemically induced mouse models of acute and chronic intestinal inflammation. Nature Protocols, 12(7), 1295–1309.
Ding, A., & Wen, X. (2018). Dandelion root extract protects NCM460 colonic cells and relieves experimental mouse colitis. Journal of Natural Medicines, 72(4), 857–866.
Huang, J., et al. (2023). Treatment of Ulcerative Colitis by Cationic Liposome Delivered NLRP3 siRNA. International Journal of Nanomedicine, 18, 4647–4662.
Zheng, S., et al. (2022). Chinese Medicine in the Treatment of Ulcerative Colitis: The Mechanisms of Signaling Pathway Regulations. The American Journal of Chinese Medicine, 50(7), 1781–1798.
Li, W. Y., et al. (2024). Sinapic Acid Attenuates Chronic DSS-Induced Intestinal Fibrosis in C57BL/6J Mice by Modulating NLRP3 Inflammasome Activation and the Autophagy Pathway. ACS Omega, 9(1), 1230–1241.
Li, Y., et al. (2024). Hypersampsonone H attenuates ulcerative colitis via inhibition of PDE4 and regulation of cAMP/PKA/CREB signaling pathway. International Immunopharmacology, 128, 111490.
Duan, B., et al. (2023). The effect and mechanism of Huangqin-Baishao herb pair in the treatment of dextran sulfate sodium-induced ulcerative colitis. Heliyon, 9(12), e23082.
McKenzie, S. J., et al. (1996). Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. Journal of Clinical Investigation, 98(1), 136–141.
Lih-Brody, L., et al. (1996). Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Digestive Diseases and Sciences, 41(10), 2078–2086.
Rahmani, S., et al. (2023). The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Science and Nutrition, 11(1), 39–56.
Kong, Y. R., et al. (2021). Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology (Basel), 10(4), 287.
Ren, G., et al. (2019). ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. The EMBO Journal, 38(6), e100376.
Cai, B., et al. (2022). USP5 attenuates NLRP3 inflammasome activation by promoting autophagic degradation of NLRP3. Autophagy, 18(5), 990–1004.
Li, X., et al. (2022). Sanguinarine ameliorates DSS induced ulcerative colitis by inhibiting NLRP3 inflammasome activation and modulating intestinal microbiota in C57BL/6 mice. Phytomedicine, 106, 154394.
Lv, Q., et al. (2021). Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharmaceutica Sinica B, 11(9), 2880–2899.
Chen, L., et al. (2022). Betaine Ameliorates Acute Sever Ulcerative Colitis by Inhibiting Oxidative Stress Induced Inflammatory Pyroptosis. Molecular Nutrition and Food Research, 66(22), e2200341.
Ginwala, R., et al. (2019). Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants (Basel), 8(2), 35.
Márquez-Flores, Y. K., et al. (2016). Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. Journal of Nutritional Biochemistry, 30, 143–152.
de Deus, I. J., et al. (2023). Role of NLRP3 inflammasome and oxidative stress in hepatic insulin resistance and the ameliorative effect of phytochemical intervention. Frontiers in Pharmacology, 14, 1188829.
Miao, F., et al. (2021). Walnut oil alleviates DSS-induced colitis in mice by inhibiting NLRP3 inflammasome activation and regulating gut microbiota. Microbial Pathogenesis, 154, 104866.
Funding
Youth Fund of Southwest Medical University, 2018-ZRQN-173. Doctor’s Fund of The Affiliated Hospital, Southwest Medical University, 18109. Science and Technology Research Special Fund of Sichuan Provincial Administration of Traditional Chinese Medicine, 2020JC0133.
Author information
Authors and Affiliations
Contributions
Xiangdong Zhao and Xiaochao Chen: Conception, design and analysis of data, performed the data analyses and wrote the manuscript; Chaochi Yue: Performed the data analyses and wrote the manuscript; All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethics Approval and Consent to Participate
The animal experiments were approved by the ethics committee The Affiliated Hospital, Southwest Medical University. All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, X., Chen, X. & Yue, C. Rutin Ameliorates Inflammation and Oxidative Stress in Ulcerative Colitis by Inhibiting NLRP3 Inflammasome Signaling Pathway. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01459-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01459-7