Abstract
Ferroptosis is a newfound mode of regulated cell death that may have potential to associate with prognostic or diagnostic factors in glioma. In this research, 5 genes related to glioma were screened through the FerrDb database, and we analyzed the combination between genes and glioma of survival and prognosis via TCGA, GEPIA, TIMER, and other databases. Survival curve and prognostic analysis showed that the overexpression of NFE2L2 and NOX4, respectively, has a remarkable link with a worse prognosis in glioma. Then, the association between the expression of the two genes and tumor-infiltrating immune cells level was explored based on the GSCA, and the immunity of NFE2L2 and NOX4 based on the TISIDB database was also investigated. In glioma, especially GBM, there is a strong association between gene expression and immune infiltration, even in macrophages, nTreg, and Th2 cells, which play immunosuppressive functions in TME. In conclusion, these results indicate that NFE2L2 and NOX4 could be risk prognosis biomarkers in glioma, and they bound up with immune infiltration and tumor immunity in tumorigenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma is a tumor caused by lesions on glial cell that is an important part of the central nervous system, which accounted for 1.6% of all new tumor cases and 2.5% of all cancer-related deaths in 2018 [1, 2]. According to the malignancy degree, gliomas are categorized into four histological grades I-IV by WHO [3]. Glioblastoma (GBM) is a type of histological grade IV glioma with only 5% of survival rate within 5 years [4]. At the present, surgical resection, chemotherapy, postoperative radiotherapy, and immunotherapy would be considered to glioma clinical treatment [5,6,7]. Nevertheless, the median survival rate of GBM patients is no more than 15 months yet [8]. Moreover, LGG also has little response to the standardized therapeutic method. Those issues, especially therapeutic resistance and tumor recurrence, lead to treatment failure and poor prognosis [9, 10]. In current clinical practice, repeatedly mutated genes such as IDH1, IDH2, TP53, EGFR, and ATRX are identified as prognostic factors in LGG [11,12,13]. Meanwhile, other molecular markers, including 1p/19q co-deletion and MGMT promoter methylation, are also considerable prognostic factors in LGG [14]. Whereas these clinicopathological and genetic factors can not accurately assess survival outcomes. Thereby, an urgent requirement for the underlying mechanism of glioma development needs to be solved, and to gain insight into the effective factors influenced the diagnosis and prognosis of glioma patients to locate new diagnostic biomarkers and find potential therapeutic targets.
Cell death is a physiological mechanism of body to maintain its normal functions through necrosis and programmed cell death (autophagy and apoptosis) [15]. The connection between cell death and disease has been illustrated after the discovery of different types of cell death such as apoptosis [16], ferroptosis [17], and pyroptosis [18]. Some reported that cell death plays a significant role in the development of gliomas, and it also affects pathological classification and sensitivity to radio-chemotherapy [19, 20].
Ferroptosis is a programmed oxidative cell death that is characterized by highly iron-dependent lipid peroxidation [17]. In recent years, ferroptosis has received widespread attention. An increasing number of pertinence between ferroptosis and neurological diseases such as cancer, stroke, and ischemia-reperfusion injury have been found [21, 22]. Moreover, recent studies have revealed that ferroptosis is a promising anticancer strategy for overcoming therapeutic resistance [23,24,25] and can be applied to a lot of anticancer regimens, and there are some of these regimens have been approved by U.S. Food and Drug Administration [26, 27]. While the pathway of diagnostic factors in ferroptosis has not been clarified yet, and there are few studies focus on ferroptosis of glioma in molecular levels. Thereby, it is valuable to lucubrate the association of ferroptosis-related genes in glioma and the prognostic value behind it.
In addition, some further studies show that cancer progression and recurrence are not only affected by the tumor’s underlying genetic changes, but also by the tumor microenvironment (TME) [28,29,30]. TME is very complex, including cellular components such as cancer cells, immune cells, endothelial cells and noncellular components such as cytokines and chemokines [31, 32]. Increasingly data sustained that immune cells in TME take a significant role in tumor progression and recurrence [33,34,35,36]. The dynamic changes of TME reflect the evolutionary nature of tumors, involving tumor immune escape, tumor growth, and metastasis. Therefore, a deeper understanding of TME and its related mechanisms are also crucial to the search of tumor prognostic factors.
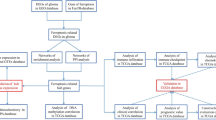
In this study, we aimed to delve into the prognostic implications of ferroptosis-related genes and immune infiltrates in glioma. Herein, we screened 5 ferroptosis-associated genes which were related to glioma through FerrDb database, and then systematically analyzed the correlation between gene expression with prognosis and immune infiltration of glioma through database systems such as TCGA, GEPIA and TISIDB. Our findings suggested that the ferroptosis-associated genes NFE2L2 and NOX4 are potential risk prognostic biomarkers and are correlated with immune infiltration in glioma.
Methods
Identification of ferroptosis-associated genes related to glioma and data collection
FerrDb is a database for preserving and providing information about ferroptosis-related markers and regulators, it also can be used to identify ferroptosis-associated diseases. We identified five ferroptosis-associated genes (Table 1) that have been tested in human glioma with different research methods in FerrDb (http://www.datjar.com:40013/bt2104/). And the ferroptosis-related signaling pathway that the five genes involved in was also analyzed.
The obtained five genes’ expression data in tumor and normal tissue samples corresponding to 33 cancer types were analyzed from TCGA (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga). And the immune histochemical images of protein expression in glioma and normal tissues were obtained from Human Protein Atlas (HPA) (https://www.proteinatlas.org/).
Survival and prognosis analysis
The relationship between gene expression and patient’s survival (OS: overall survival; DFS: disease-free survival) in GBM and LGG were obtained from GEPIA(http://gepia.cancer-pku.cn/) and TIMER (https://cistrome.shinyapps.io/timer/). Then, the relationship between gene expression and glioma histology was analyzed from the TCGA database. The prognostic ability was evaluated by ROC and AUC analysis using the package of “survival ROC” in R. We also conducted multivariate Cox proportional hazards model analysis to evaluate whether a gene can be used as an independent prognostic factor in GBM and LGG using the Timer database. The hazard ratio (HR) and 95% confidence intervals were calculated via univariate survival analysis.
Correlations between gene expression and immunogenic features in glioma
Gene Set Cancer Analysis (GSCA, http://bioinfo.life.hust.edu.cn/GSCA/#/) is an integrated platform for genomic, pharmacogenomic, and immunogenomic gene set cancer analysis. The immune infiltration levels were quantified using GSCA, which provided immunogenomic analysis was performed by the ImmuCellAI algorithm on 24 immune cells. We explored the correlation analysis between gene expression and immune infiltration levels in glioma (GBM and LGG). The heatmap and spearman correlation analysis between gene expression and immunity in glioma (GBM and LGG) were analyzed from TISIDB (http://cis.hku.hk/TISIDB/).
Statistical analysis
Performing statistical analyses with R software and SPSS software. By long-rank test, the correlation between critical factors and overall survival rate of patients had been analyzed that based on the survival and survminer R package. Spearman was applied to identify the relevance of genes and immune infiltration. Data were considered significant at p < 0.05.
Results
Identification of 5 ferroptosis-associated genes
We screened ferroptosis-associated genes related to glioma through the FerrDb database. As shown in Table 1, there were 5 ferroptosis-associated genes (KEAP1, NFE2L2, NOX4, ATF4, ASCL4) that have been tested in human glioma with different research methods. As shown in Fig. S1, 5 genes play an important role in the process of ferroptosis through different signaling pathways. Kelch like ECH associated protein1 (KEAP1) was defined as a significant factor that regulates the antioxidant response pathway and restrains erythroid-1 like 2 (NFE2L2/NRF2) which is a transcription factor [37]. While, NFE2L2 is the key factor that manages lots of cell protection genes in transcription, which genes involved in iron metabolism, oxidative defense, and redox signaling during ferroptosis [38]. And NADPH oxidase (NOX) is a family of enzymes that produce ROS. Studies have shown that NOX4, as the main isotype in astrocytes, can promote the ferroptosis of astrocytes through lipid peroxidation induced by oxidative stress [39, 40]. Acyl-CoA synthetase long-chain family member 4 (ACSL4), catalyze fatty acid metabolism, has the potential to be a biomarker of specificity and promoter of ferroptosis since the ACSL4 overexpression can increase PUFA content in phospholipids [41,42,43,44]. Activating transcription factor 4 (ATF4) is an essential link between ER stress and oxidative damage, contributing to ferroptosis in a tumor-type-dependent manner mediated by a signal pathway such as ATF4–HSPA5–GPX4 [45, 46].
Prognostic significance of ferroptosis-associated genes in glioma
To investigate if ferroptosis-related genes are associated with the progression of glioma, we firstly obtained the gene expression data in human pancancer from the GEPIA database. As shown in Fig. S2, the expression of KEAP1, NFE2L2, and NOX4 were generally higher than normal level of body in more than 20 tumor tissue samples. However, ATF4 showed reversed expression trends with low expression in most tumor tissues compared to normal tissues. As for the expression in glioma, the results are shown in Fig. 1, KEAP1 and NFE2L2 showed a near 2~3 fold increase in both GBM and LGG, and the NOX4 showed a nearly 20-fold increase in GBM. While the expression of ATF4 and ASCL4 did not be significantly different in GBM and LGG compared to normal tissues. Then, the Human Protein Atlas (HPA) database analysis (Fig. 1B) also shows that KEAP1 and NFE2L2 expression is significantly higher in glioma tissues compared to normal tissues.
mRNA and protein expression of ferroptosis-associated genes in glioma and normal tissues. A Profile and box plots show gene mRNA expression in GBM/LGG (red) and normal tissue samples, *p < 0.05; B Immuno-histochemical images from HPA database show KEAP1 and NFE2L2 protein expression in glioma and normal tissues
The ferroptosis-related genes expression analysis showed the relationship of KEAP1, NFE2L2, and NOX4 expression with patients’ survival in GBM and LGG, and disease-free survival (DFS) and overall survival (OS) were performed by GEPIA (Fig. 2A). Notably, the DFS and OS were significantly shorter in patients with high expression of NFE2L2 and NOX4 than in those with low expression of NFE2L2 and NOX4. However, KEAP1 expression was ineffective association with the survival curve. This result implied that NFE2L2 and NOX4 could be the key product that caused poor prognosis in glioma patients. Furthermore, we analyzed the correlation of 1, 3, and 5-year cumulative survival rates of glioma patients with NFE2L2 and NOX4 expression. As shown in Fig. 2B, the cumulative survival of glioma patients with low NFE2L2 or NOX4 expression was significantly longer than that of glioma patients with high expression. And the results also showed that the HR value of the survival curve gradually increased with time lapse, which means that the mortality of glioma patients with high NOX4 expression gradually increased with the prolongation of time. In conclusion, the eccentric expression and appearance of NFE2L2 and NOX4 are risk factors in glioma.
Relationship of ferroptosis-related gene expression with patients’ survival in GBM and LGG. A Disease free survival and overall survival curves of KEAP1, NFE2L2, and NOX4 in glioma patients were analyzed by GEPIA. B 1, 3, 5-year cumulative survival rate of glioma patients correlated with NFE2L2 and NOX4 expression analyzed from TIMER database
From the result of silico analysis by the TCGA dataset, it showed that IDH mutation and chromosome 1p/19q co-deletion are critical biomarkers that direct treatment and prognostication of glioma [47]. We found that low expression of NFE2L2 and NOX4 was associated with IDH mutated and 1p/19q codeletion group (Fig. 3A, B). Besides, the expression of NFE2L2 and NOX4 was positively correlated with the WHO grade of glioma (Fig. 3C). This means that the expression levels of NFE2L2 and NOX4 would increase with the glioma development. The analysis results (Fig. 3D) also showed that the two-gene expression was related to histology. As shown in Fig. 3D, the expression of NFE2L2 increases with the histological severity of glioma, and NOX4 is also significantly increased in the gradeIV glioblastoma. Besides, according to scores of pathological features, prognostic values were determined from the ROC curves by calculating the area under the curve (AUCs) of the gene expression. We observed that the prognostic value of those two genes in gliomas AUCs are 0.929 and 0.813, respectively (Fig. 3E).
The association of gene expression (FPKM) with glioma histology and prognostic biomarkers in the TCGA database. A–D Correlation of NFE2L2 and NOX4 expression with (A) 1p/19q codeletion status, (B) IDH mutation status, (C) Grade and (D) Histology. *p < 0.05, **p < 0.01, ***p < 0.001. E ROC curve showed the predictive efficiency of gene expression
To further determine whether gene expression represented an independent prognostic index in glioma, Cox proportional hazard model analyses of NFE2L2 and NOX4 mRNA levels and clinical factors predictive of overall survival in GBM and LGG were performed using the Timer database. As shown in Fig. 4, there is only age that is considered as a risk factor (P < 0.001, Hazard Ratio, HR > 1) that significantly affected prognosis in GBM. However, in addition to age, NFE2L2 and NOX4 can significantly affect the prognosis in LGG. On the other side, the HR values of NFE2L2 and NOX4 were 3.12 and 1.76, respectively, indicating that these two genes are the risk factors that affect the prognosis of LGG. Thereby, the risk of death is in direct proportion to the expression of the two genes. The above results proved that NFE2L2 and NOX4 can be used as prognostic risk factors in gliomas, especially in low-grade gliomas.
The interaction of tumors and immune cells will facilitate the anticipation of immunotherapy response and the development of novel immunotherapy targets. The interaction between tumors and immune cells plays a crucial role in the occurrence, development, and treatment of cancer. Besides, tumor-infiltrating immune cell is an independent predictor of cancer status and patients’ OS [48]. The affiliation of gene expression and immune infiltration levels in glioma was probed by using GSCA in this study. Our results showed the correlation of NFE2L2 expression level with high immune infiltration both in GBM and LGG, while NOX4 expression mainly correlated with high immune infiltration in LGG (Fig. 5). These analyses also revealed that high expression levels of NFE2L2 could significantly increase the immune infiltrating levels of exhausted T cells, macrophages, monocyte cells, NKT, Tfh, Th17, Th2, and nTreg cells, especially of macrophages (Cor. = 0.46) and nTreg cells (Cor. = 0.26), while passive tie was detected for levels of CD8 naïve T Cells (Cor. = −0.37), B cells (Cor. = −0.35), CD4 naïve T cells and Gamma delta T cells in GBM (Fig. 5A, Fig S3A). In LGG, the relationships between NFE2L2 expression and immune infiltration levels were a little different (Fig. 5B, Fig. S3B). NFE2L2 expression was positively associated with cytotoxic cells, DC, exhausted T cells, MAIT, macrophage (Cor. = 0.42), monocyte cells, Th1, Th17, and Th2 (Cor. = 0.36), while negatively associated with B cells, CD4 T cells, CD4 naïve, CD8 T cells, CD8 naïve cells, NKT (Cor. = −0.14) and neutrophil cells (Cor. = −0.16). Similarly, as shown in Fig. 5C and Fig. S4C, NOX4 expression levels were positively associated with 4 types of infiltrating immune cells, including cytotoxic T cells, macrophages (Cor. = 0.23), monocyte cells (Cor. = 0.26) and NKT cells (Cor. = 0.24) and negatively associated with 3 types of infiltrating immune cells, including CD4 T cells, CD4 naïve T cells (Cor. = −0.27) and CD8 T cells in GBM. However, in LGG, high NOX4 expression could meaningfully enhance the infiltrating levels of many kinds of T cells including CD8 T cells, cytotoxic T cells, DC, effector memory cells, gamma delta T cells, MAIT, Tfh, Th1, Th2, Tr1, and nTreg cells. Moreover, a negative correlation exists between the NOX4 expression level and infiltration levels of CD4 T cells and neutrophil cells (Fig. 5D, Fig. S3D).
To analyze the association of the two genes and immunogenic features more fully, we validated the correlation of NFE2L2 and NOX4 expression with cytokines, MHC, receptor, immune-inhibitor and immune stimulator in GBM and LGG, respectively. As shown in Fig. 6 and Fig. S4, among the 166 GBM samples and 166 LGG samples analyzed for cytokine and MHC expression, the expression of CCL2 and HLA-E was most significantly positively correlated with the NFE2L2. For receptors, immuno-inhibitors, and immuno-stimulators, the expression of immune factors is different in GBM and LGG. In GBM, CXCR4 and PSCD1LG2 were positively correlated with NFE2L2, and TNFRSF13C was most significantly negatively correlated. While in LGG, CCR1 and IL-6R were positively correlated with NFE2L2, CD160 was negatively correlated. As for the NOX4, the results showed that TNFRSF4 expression of immuno-stimulators both in GBM and LGG was most significantly positively correlated with gene expression. However, in GBM, the chemokines of CXCL8, a receptor of CXCR1, an immuno-inhibitor of KDR, and MHC of HLA-A were positively correlated with NOX4. While in LGG, the chemokines of CXCL10, the receptor of CXCR4, an immuno-inhibitor of IL-10RB, and MHC of TAP1 were most significantly positively correlated with NOX4.
Discussion
Ferroptosis is a newly found programmed cell death that relies on iron ion that can promote lipid peroxidation, which is different with apoptosis or necrosis [17]. The former researches have explored that it is common that high levels of iron are related in a variety of cancer cells rather than in normal cells [49,50,51,52,53]. Moreover, cell death, especially ferroptosis, is critical for glioma development, and it affects pathological classification and sensitivity to chemoradiotherapy. Therefore, cell death is a very promising anticancer strategy to overcome treatment resistance [23,24,25]. Temozolomide is the most widely used chemical in glioma treatment and has been reported to kill tumor cells through various pathways, including ferroptosis [15, 54, 55]. According to the above evidence, ferroptosis-related drugs with temozolomide could prolong PFS and even OS in the treatment of glioma. As such, ferroptosis is a potential novel approach for glioma treatment. Therefore, it is expressive to analyze the association of ferroptosis-related genes in glioma patients and their prognostic value.
In this study, we first identified 5 ferroptosis-related genes that are closely related to glioma based on the Ferrbe database. Then, we explored the prognostic significance of the 5 ferroptosis-associated genes in glioma by analyzing the gene expression and survival curve of glioma patients. Our results verify that KEAP1 and NFE2L2 display clearly higher expression in glioma (GBM and LGG) tissues than in adjacent normal tissues. And the NOX4 showed a nearly 20-fold increase only in GBM. Furthermore, disease-free survival (DFS) and overall survival (OS) were performed by GEPIA (Fig. 2A). Notably, the DFS and OS were significantly shorter in patients with high expression of NFE2L2 and NOX4 than in those with low expression. However, KEAP1 expression was not significantly associated with the survival curve.
In 2016 WHO categorization, codeletion of chromosomal arms 1p/19q (1p/19q codeletion) and isocitrate dehydrogenase 1 or 2 (IDH1 or IDH2) were included in the diagnostic typing for glioma classification [2, 56]. Recurrently mutated genes IDH1, IDH2, and 1p/19q are prominent identified factors in prognosis of LGGs in clinical application [11,12,13]. Our analysis proved that low NFE2L2 and NOX4 expression in glioma patients from the TCGA database were significantly related to better pathologic stage and histological grade (Fig. 3). More importantly, we observed that NFE2L2 and NOX4 expression have a prognostic value in glioma with AUCs of 0.929 and 0.813, respectively. All results revealed that NFE2L2 and NOX4 appeared to be risk factors in glioma. This indicates that the NFE2L2 and NOX4 expression-based signatures can better predict the prognosis of glioma.
Tumor microenvironment (TME), the place for tumor birth and growth, play a meaningful role in cancer diagnosis and determining therapeutic options. Moreover, lots of current researches have intimated that the curative effect of immunotherapy is related to the immunogenic TME [32, 57]. Thus, a recondite understanding of the TME in glioma could contribute to digging new ominous markers. We further analyzed the correlation between expression of NFE2L2 and NOX4 and immunogenic features, respectively. We found that NFE2L2 was positively correlated with macrophages and nTreg cells and destructively interrelated with B cells and CD8 naïve cells in GBM. Similarly, NFE2L2 expression was specifically related to macrophage and Th2 cells and negatively correlated with CD8 naïve and NKT cells in LGG. While NOX4 expression levels were significantly positively associated with macrophage T cells and negatively correlated with CD4 T cells in GBM. However, in LGG, the high expression levels of NOX4 could significantly increase gamma delta T cells, Th1, and cytotoxic T cells. Although macrophages have the potential to kill tumors, tumor cells can promote tumor cell expansion and growth by binding to SIRPa on the surface of macrophages in tumor-infiltrating areas through high expression of CD47 [58]. Besides, nTreg and Th2 cells play immunosuppressive functions in TME. However, immune cells such as CD8, NKT, and CD4 play important antitumor immune effects in the TME. These observations suggest that the glioma microenvironment with high expression of NFE2L2 or NOX4 was in an immunosuppressive state that confirmed immune system is meaningful to glioma development.
In addition, we validated the correlation of NFE2L2 and NOX4 expression with immune cytokines, MHC receptors, immune inhibitors, and activators in GBM and LGG to acquire an extensive understanding of the association between gens and immunogenicity signatures. Interestingly, in GBM, NFE2L2 expression was positively correlated with many MHCs, cytokines, and other immune molecules such as CCL2, HLA-E, and CXCR4. While in LGG, more immune molecules were positively associated with NFE2L2 expression, such as CCL2, CCR1, and IL-6R. Similarly, in GBM and LGG, NOX4 expression was positively correlated with more than 50 immune molecules, such as TNFRSF4, CXCL8, HLA-A, and IL-10RB. Collectively, all results further strongly indicate that NFE2L2 and NOX4 play important roles in tumor immunity.
In summary, we systematically investigated the expression patterns of ferroptotic gene in glioma and the relationship to patient outcome. The results of current research point that the overexpression of NFE2L2 and NOX4 correlates with poor prognosis in clinical treatment, particularly in LGG. In addition, the high expression of NFE2L2 or NOX4 was in an immunosuppressive state, and it correlated with tumor immunity in glioma. Therefore, NFE2L2 and NOX4 may function as a risk prognosis biomarker for glioma and play a vital role in immune infiltration and tumor immunity.
References
Bray, F. et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424.
Zhuo, S. et al. (2020). Clinical and biological significances of a ferroptosis-related gene signature in glioma. Frontiers in Oncology, 10, 590861
Wesseling, P. & Capper, D. (2018). WHO 2016 Classification of gliomas. Neuropathology and Applied Neurobiology, 44, 139–150.
Liu, G. et al. (2018). Survival analysis for high-grade glioma patients who received comprehensive treatment. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 43(4), 388–393..
Jiang, T. et al. (2016). CGCG clinical practice guidelines for the management of adult diffuse gliomas. Cancer Letters, 375(2), 263–273.
Jhaveri, J. et al. (2018). Is less more? Comparing chemotherapy alone with chemotherapy and radiation for high-risk grade 2 glioma: An analysis of the National Cancer Data Base. Cancer, 124(6), 1169–1178.
Xu, S. et al. (2020). Immunotherapy for glioma: Current management and future application. Cancer Letters, 476, 1–12.
Morgan, L. L. (2015). The epidemiology of glioma in adults: a “state of the science” review. Neuro-Oncology, 17(4), 623–624.
Andersen, B. M. et al. (2019). Leptomeningeal metastases in glioma: The Memorial Sloan Kettering Cancer Center experience. Neurology, 92(21), e2483–e2491.
Lv, S. et al. (2020). The prognostic role of glutathione peroxidase 1 and immune infiltrates in glioma investigated using public datasets. Medical Science Monitor, 26, e926440.
Sun, Y. et al. (2014). A glioma classification scheme based on coexpression modules of EGFR and PDGFRA. Proceedings of the National Academy of Sciences USA, 111(9), 3538–3543.
Liu, X. Y. et al. (2012). Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathologica, 124(5), 615–625.
Yan, H. et al. (2009). IDH1 and IDH2 mutations in gliomas. New England Journal of Medicine, 360(8), 765–773.
Weller, M. et al. (2012). Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro-Oncology, 14, iv100–iv108.
Liu, Y. et al. (2020). Ferroptosis in low-grade glioma: a new marker for diagnosis and prognosis. Medical Science Monitor, 26, e921947.
Vanden Berghe, T. et al. (2014). Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature Reviews Molecular Cell Biology, 15(2), 135–147.
Dixon, S. J. et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 149(5), 1060–1072.
Cookson, B. T. & Brennan, M. A. (2001). Pro-inflammatory programmed cell death. Trends in Microbiology, 9(3), 113–114.
Wechsler-Reya, R. & Scott, M. P. (2001). The developmental biology of brain tumors. Annual Review of Neuroscience, 24, 385–428.
Schiffer, D. et al. (2018). Glioblastoma: microenvironment and niche concept. Cancers (Basel), 11(1), 5–23.
Yang, W. S. & Stockwell, B. R. (2016). Ferroptosis: death by lipid peroxidation. Trends in Cell Biology, 26(3), 165–176.
Friedmann Angeli, J. P. et al. (2014). Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biology, 16(12), 1180–1191.
Wu, J. et al. (2019). Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature, 572(7769), 402–406.
Viswanathan, V. S. et al. (2017). Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature, 547(7664), 453–457.
Jiang, L. et al. (2015). Ferroptosis as a p53-mediated activity during tumour suppression. Nature, 520(7545), 57–62.
Sui, X. et al. (2018). RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Frontiers in Pharmacology, 9, 1371.
Eling, N. et al. (2015). Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience, 2(5), 517–532.
McAllister, S. S. & Weinberg, R. A. (2014). The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nature Cell Biology, 16(8), 717–727.
Quail, D. F. & Joyce, J. A. (2013). Microenvironmental regulation of tumor progression and metastasis. Nature Medicine, 19(11), 1423–1437.
Yang, I. et al. (2011). Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. Journal of Neurosurgery, 115(3), 505–511.
Chen, Z. & Hambardzumyan, D. (2018). Immune microenvironment in glioblastoma subtypes. Frontiers in Immunology, 9, 1004
Xu, W. H. et al. (2019). Prognostic value and immune infiltration of novel signatures in clear cell renal cell carcinoma microenvironment. Aging (Albany NY), 11(17), 6999–7020.
Grivennikov, S. I., Greten, F. R. & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140(6), 883–899.
Elinav, E. et al. (2013). Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nature Reviews Cancer, 13(11), 759–771.
Song, L.R. et al. (2020). Prognostic and predictive value of an immune infiltration signature in diffuse lower-grade gliomas. JCI Insight, 5(8), 13381–13396.
Xiao, K. et al. (2020). Prognostic value and immune cell infiltration of hypoxic phenotype-related gene signatures in glioblastoma microenvironment. Journal of Cellular and Molecular Medicine, 24(22), 13235–13247.
Rojo de la Vega, M., Chapman, E. & Zhang, D. D. (2018). NRF2 and the Hallmarks of Cancer. Cancer Cell, 34(1), 21–43.
Tang, D. et al. (2021). Ferroptosis: molecular mechanisms and health implications. Cell Research, 31(2), 107–125.
Nayernia, Z., Jaquet, V. & Krause, K. H. (2014). New insights on NOX enzymes in the central nervous system. Antioxidants & Redox Signaling, 20(17), 2815–2837.
Park, M. W. et al. (2021). NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biology, 41, 101947.
Yuan, H. et al. (2016). Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochemical and Biophysical Research Communications, 478(3), 1338–1343.
Doll, S. et al. (2017). ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology, 13(1), 91–98.
Kagan, V. E. et al. (2017). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nature Chemical Biology, 13(1), 81–90.
Yang, W. S. et al. (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proceedings of the National Academy of Sciences, 113(34), E4966–E4975.
Zhu, S. et al. (2017). HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Research, 77(8), 2064–2077.
Dai, C. et al. (2020). Transcription factors in ferroptotic cell death. Cancer Gene Therapy, 27(9), 645–656.
Eckel-Passow, J. E. et al. (2015). Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. New England Journal of Medicine, 372(26), 2499–2508.
Azimi, F. et al. (2012). Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. Journal of Clinical Oncology, 30(21), 2678–2683.
Shoja, Z. et al. (2019). Role of iron in cancer development by viruses. Reviews in Medical Virology, 29(4), e2045.
Torti, S. V. & Torti, F. M. (2013). Iron and cancer: more ore to be mined. Nature Reviews Cancer, 13(5), 342–355.
Liu, Y. et al. (2020). Development and validation of a combined ferroptosis and immune prognostic classifier for hepatocellular carcinoma. Frontiers in Cell and Developmental Biology, 8, 596679.
Torti, S. V. et al. (2018). Iron and cancer. Annual Review of Nutrition, 38, 97–125.
Zhu, X. et al. (2021). Characterization of ferroptosis signature to evaluate the predict prognosis and immunotherapy in glioblastoma. Aging (Albany NY), 13(13), 17655–17672.
Sehm, T. et al. (2016). Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget, 7(46), 74630–74647.
Buccarelli, M. et al. (2018). Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death & Disease, 9(8), 841.
Louis, D. N. et al. (2016). The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica, 131(6), 803–820.
Reap, E. A. et al. (2018). Dendritic cells enhance polyfunctionality of adoptively transferred T cells that target cytomegalovirus in glioblastoma. Cancer Research, 78(1), 256–264.
Weiskopf, K. & Weissman, I. L. (2015). Macrophages are critical effectors of antibody therapies for cancer. MAbs, 7(2), 303–310.
Funding
The authors sincerely thank all participants involved in this study. The study was supported by the National Natural Science Foundation of China (32000534).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, L., Li, X., Zhu, S. et al. Ferroptosis-related NFE2L2 and NOX4 Genes are Potential Risk Prognostic Biomarkers and Correlated with Immunogenic Features in Glioma. Cell Biochem Biophys 81, 7–17 (2023). https://doi.org/10.1007/s12013-022-01124-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-022-01124-x