Abstract
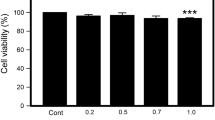
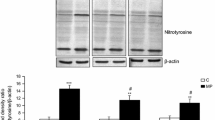
2-Hexadecenal (2-HD)—a biologically active long-chain fatty aldehyde formed in organism enzymatically or nonenzymatically in the reaction of free-radical destruction of sphingolipids under the action of hypochlorous acid, producing by myeloperoxidase. This research aimed to study 2-HD effects on polymorphonuclear leukocytes’ (PMNLs) functions. It has been shown that at submicromolar concentrations, 2-HD causes an elevation in ROS production by PMNLs. It has been found that such effect is associated with signal transduction pathways modification and expressed in elevation of NADPH oxidase, MPO, and JNK-MAPK contributions to this process. At higher concentrations, 2-HD induces apoptosis, which correlates with a significant increase in free Ca2+ in the cytoplasm, a decrease in ROS production, and a decline in mitochondrial potential. Both of these processes are accompanied by cytoskeleton reorganization.







Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bartke, N., & Hannun, Y. A. (2009). Bioactive sphingolipids: metabolism and function. Journal of Lipid Research, Suppl 50, S91–S96.
An, S., Zheng, Y., & Bleu, T. (2000). Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. Journal of Biological Chemistry, 275, 288–296.
Harada, J., Foley, M., Moskowitz, M. A., & Waeber, C. (2004). Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. Journal of Neurochemistry, 88, 1026–1039.
Pyne, N. J., & Pyne, S. (2010). Sphingosine 1-phosphate and cancer. Nature Review Cancer, 10, 489–503.
Kumar, A., Byun, H.-S., Bittman, R., & Saba, J. D. (2011). The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell Signaling, 23, 1144–1152.
Amaegberi, N. V., Semenkova, G. N., Kvacheva, Z. B., Lisovskaya, A. G., Pinchuk, S. V., & Shadyro, O. I. (2019). 2-Hexadecenal inhibits growth of C6 glioma cells. Cell Biochemistry & Function, 37, 281–289.
Ebenezer, D. L., Fu, P., Ramchandran, R., Ha, A. W., Putherickal, V., Sudhadevi, T., Harijith, A., Schumacher, F., Kleuser, B., & Natarajan, V. (2020). S1P and plasmalogen derived fatty aldehydes in cellular signaling and functions. Biochimica et Biophysica Acta, 1865, 158681.
Amaegberi, N. V., Semenkova, G. N., Lisovskaya, A. G., Kvacheva, Z. B., & Shadyro, O. I. (2019). Modification of redox processes in C6 glioma cells by 2-hexadeсenal, the product of sphingolipid destruction. Biophysica (Russian Federation), 64, 424–430.
Ebenezer, D. L., Ramchandran, R., Fu, P., Mangio, L. A., Suryadevara, V., Ha, A. W., Berdyshev, E., Van Veldhoven, P. P., Kron, S. J., Schumacher, F., Kleuser, B., & Natarajan, V. (2021). Nuclear sphingosine-1-phosphate lyase generated ∆2-hexadecenal is a regulator of HDAC activity and chromatin remodeling in lung epithelial cells. Cell Biochemistry and Biophysics, 79(3), 575–592.
Brahmbhatt, V. V., Hsu, F. F., Kao, J. L. F., Frank, E. C., & Ford, D. A. (2007). Novel carbonyl and nitrile products from reactive chlorinating species attack of lysosphingolipid. Chemistry and Physics of Lipids, 145, 72–84.
Lisovskaya, A. G., Shadyro, O. I., & Edimecheva, I. P. (2011). A new mechanism for photo- and radiation-induced decomposition of sphingolipids. Lipids, 46, 271–276.
Lisovskaya, A. G., Edimecheva, I. P., & Shadyro, O. I. (2012). A novel pathway of photoinduced decomposition of sphingolipids. Photochemistry and Photobiology, 88(4), 899–903.
Shadyro, O., Lisovskaya, A., Semenkova, G., Edimecheva, I., & Amaegberi, N. (2015). Free-radical destruction of sphingolipids resulting in 2-hexadecenal formation. Lipid Insights, 8, 1–9.
Shadyro, O., & Lisovskaya, A. (2019). ROS-induced lipid transformations without oxygen participation. Chemistry and Physics of Lipids, 221, 176–183.
Lisovskaya, A., Pratsenka, K., Kulinkina, A., Semenkova, G. N., & Shadyro, O. (2015). Sphingolipid destruction in HOCl-treated red blood cellsle. FEBS Journal, 282, 235.
Arnhold, J. (2020). The dual role of myeloperoxidase in immune response. International Journal of Molecular Science, 21(21), 8057.
Babior, B. M. (2000). Phagocytes and oxidative stress. The American Journal of Medicine, 109, 33–44.
Kolaczkowska, E., & Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology, 13, 159–175.
Leick, M., Azcutia, V., Newton, G., & Luscinskas, F. W. (2014). Leukocyte recruitment in inflammation: Basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell and Tissue Research, 355, 647–656.
Kettle, A. J., & Winterbourn, C. C. (1997). Myeloperoxidase: A key regulator of neutrophil oxidant product. Redox Report, 3(1), 3–15.
Kuznetsova, T., Kulahava, T., Zholnerevich, I., Amaegberi, N., Semenkova, G., Shadyro, O., & Arnhold, J. (2017). Morphometric characteristics of neutrophils stimulated by adhesion and hypochlorite. Molecular Immunology, 87, 317–324.
Brahmbhatt, V. V., Albert, C. J., Anbukumar, D. S., Cunningham, B. A., Neumann, W. L., & Ford, D. A. (2010). ω-oxidation of α-chlorinated fatty acids: Identification of α-chlorinated dicarboxylic acids. Journal of Biological Chemistry, 285, 41255–41269.
Hawkins, C. L. (2019). Hypochlorous acid-mediated modification of proteins and its consequences. Essays in Biochemistry, 64, 75–86.
Thukkani, A. K., Hsu, F. F., Crowley, J. R., Wysolmerski, R. B., Albert, C. J., & Ford, D. A. (2002). Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: Production of the chemoattractant, 2-chlorohexadecanal. Journal of Biological Chemistry, 277, 3842–3849.
Amaegberi, N. V., Semenkova, G. N., Lisovskaya, A. G., Gusakova, S. S., Prokasheva, V. А, & Shadyro, O. I. (2019). Investigation of the regulatory effect of 2-hexadecenal on neutrophils by the chemiluminescence method. Journal of Applied Spectroscopy, 86, 636–642.
Liu, Z., Gong, Y., Byun, H. S., & Bittman, R. (2010). An improved two-step synthetic route to primary allylic alcohols from aldehydes. New Journal of Chemistry, 34, 470–475.
Bøyum, A. (1976). Isolation of lymphocytes, granulocytes and macrophages. Scandinavian Journal of Immunology, Suppl 5, 9–15.
Kato, F., Tanaka, M., & Nakamura, K. (1999). Rapid fluorometric assay for cell viability and cell growth using nucleic acid staining and cell lysis agents. Toxicology in Vitro, 13, 923–929.
Liu, L., Dahlgren, C., Elwing, H., & Lundqvist, H. (1996). A simple chemiluminescence assay for the determination of reactive oxygen species produced by human neutrophils. Journal of Immunology, 192, 173–178.
Semenkova, G. N., Cherenkevich, S. N., Levin, V. I., & Svirnovskiĭ, A. I. (1985). Generation of active forms of oxygen by human peripheral blood neutrophils and lymphocytes during adhesion to glass. Biofizika, 30, 864–867.
Kavalenka, A. I., Semenkova, G. N., & Cherenkevich, S. N. (2007). Effects of hydrogen peroxide on neutrophil ability to generate reactive oxygen and chlorine species and to secrete myeloperoxidase in vitro. Cell and Tissue Biology, 1, 551–559.
Cui, X., Zhang, X., Yin, Q., Meng, A., Su, S., Jing, X., Li, H., Guan, X., Li, X., Liu, S., & Cheng, M. (2014). F‑actin cytoskeleton reorganization is associated with hepatic stellate cell activation. Molecular Medicine Reports, 9, 1641–1647.
Sivandzade, F., Bhalerao, A., & Cucullo, L. (2019). Analysis of the mitochondrial membrane potential using the cationic JC-1 Dye as a sensitive fluorescent probe. Bio-Protocol, 9, 1–13.
Patel, A., Hirst, R. A., Harrison, C., Hirota, K., & Lambert, D. G. (2013). Measurement of [Ca2+]i in whole cell suspensions using fura-2. Methods in Molecular Biology, 937, 37–47.
Ishaque, A., & Al-Rubeai, M. (2007). Measurement of apoptosis in cell culture. In R. Pörtner ed, Animal cell biotechnology. Methods in biotechnology (pp. 285–299). Totowa, NJ: Humana.
Görlach, A., Bertram, K., Hudecova, S., & Krizanova, O. (2015). Calcium and ROS: a mutual interplay. Redox Biology, 6, 260–271.
Pinton, P., Giorgi, C., Siviero, R., Zecchini, E., & Rizzuto, R. (2008). Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene, 27, 6407–6418.
McCracken, J. M., & Allen, L. A. H. (2014). Regulation of human neutrophil apoptosis and lifespan in health and disease. Journal of Cell Death, 7, 15–23.
Bengtsson, T., Orselius, K., & Wetterö, J. (2006). Role of the actin cytoskeleton during respiratory burst in chemoattractant-stimulated neutrophils. Cell Biology International, 30, 154–163.
Lakshman, R., & Finn, A. (2001). Neutrophil disorders and their management. Journal of Clinical Pathology, 54, 7–19.
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., & Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling, 20, 1126–1167.
Nauseef, W. M. (2004). Assembly of the phagocyte NADPH oxidase. Histochemistry and Cell Biology, 122, 277–291.
Panday, A., Sahoo, M. K., Osorio, D., & Batra, S. (2015). NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cellular & Molecular Immunology, 12, 5–23.
Herlaar, E., & Brown, Z. (1999). p38 MAPK signalling cascades in inflammatory disease. Molecular Medicine Today, 10, 439–447.
Mócsai, A., Jakus, Z., Vántus, T., Berton, G., Lowell, C. A., & Ligeti, E. (2000). Kinase pathways in chemoattractant-induced degranulation of neutrophils: The role of p38 mitogen-activated protein kinase activated by Src family kinases. Journal of Immunology, 164, 4321–4331.
Dhanasekaran, D. N., & Reddy, E. P. (2008). JNK signaling in apoptosis. Oncogene, 27, 6245–6251.
Son, Y., Cheong, Y.-K., Kim, N.-H., Chung, H.-T., Kang, D. G., & Pae, H.-O. (2011). Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? Journal of Signal Transduction, 2011, 1–6.
Cohen, D. T., Wales, T. E., McHenry, M. W., Engen, J. R., & Walensky, L. D. (2020). Site-dependent cysteine lipidation potentiates the activation of proapoptotic BAX. Cell Reports, 30(10), 3229–3239.
Bernhart, E., Kogelnik, N., Prasch, J., Gottschalk, B., Goeritzer, M., Depaoli, M. R., Reicher, H., Nusshold, C., Plastira, I., Hammer, A., Fauler, G., Malli, R., Graier, W. F., Malle, E., & Sattler, W. (2018). 2-Chlorohexadecanoic acid induces ER stress and mitochondrial dysfunction in brain microvascular endothelial cells. Redox Biology, 15, 441–451.
Messner, M. C., Albert, C. J., & Ford, D. A. (2008). 2-Chlorohexadecanal and 2-chlorohexadecanoic acid induce COX-2 expression in human coronary artery endothelial cells. Lipids, 43, 581–588.
Bengtsson, T., Orselius, K., & Wetterö, J. (2006). Role of the actin cytoskeleton during respiratory burst in chemoattractant-stimulated neutrophils. Cell Biology International, 30, 154–163.
Stanley, A., Thompson, K., Hynes, A., Brakebusch, C., & Quondamatteo, F. (2014). NADPH oxidase complex-derived reactive oxygen species, the actin cytoskeleton, and rho GTPases in cell migration. Antioxidants & Redox Signaling, 20, 2026–2042.
Ayub, K., & Hallett, M. B. (2004). Ca2+ influx shutdown during neutrophil apoptosis: importance and possible mechanism. Immunology, 111, 8–12.
Bagur, R., & Hajnóczky, G. (2017). Intracellular Ca2+ sensing: role in calcium homeostasis and signaling. Physiology & Behavior, 176, 139–148.
Pinton, P., Giorgi, C., Siviero, R., Zecchini, E., & Rizzuto, R. (2008). Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene, 27, 6407–6418.
Kim-Park, W. K., Moore, M. A., Hakki, Z. W., & Kowolik, M. J. (1997). Activation of the neutrophil respiratory burst requires both intracellular and extracellular calcium. Annals of the New York Academy of Sciences, 832, 394–404.
Funding
This study was funded by the Ministry of Education of Belarus [grant number 20180589] and the World Federation of Scientists.
Author information
Authors and Affiliations
Contributions
Galina Semenkova and Oleg Shadyro conceived and designed the experimental study. Nadezda Amaegberi, Alexandra Lisovskaya, Anna Poleshko and Serge Pinchuk performed the experiments, acquired and analyze data. All authors participated in the writing of manuscript. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no Competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Semenkova, G.N., Amaegberi, N.V., Lisovskaya, A.G. et al. 2-Hexadecenal Regulates ROS Production and Induces Apoptosis in Polymorphonuclear Leucocytes. Cell Biochem Biophys 81, 77–86 (2023). https://doi.org/10.1007/s12013-022-01117-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-022-01117-w