Abstract
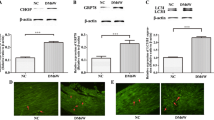
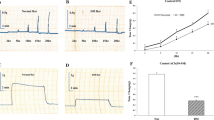
This study aimed to investigate the effect of AMPK on apoptosis and energy metabolism of gastric smooth muscle cells in diabetic rats and to explore the role of AMPK in the pathogenesis of diabetic gastroparesis (DGP). After establishment of a diabetic rat model, rats were divided into normal control (NC), 4-week (DM4W), 6-week (DM6W), and 8-week (DM8W) diabetic model groups. The gastric residual pigment ratio, intestinal transit rate, and intestinal propulsion rate in each group were detected to confirm the successful establishment of the DGP model. The spontaneous contraction in isolated gastric smooth muscle strips of the NC and DM8W groups was experimentally observed. The expression of phospho-AMPK, AMPK, phospho-LKB1, LKB1, phospho-TAK1, TAK1, and CaMMKβ in rat gastric smooth muscle tissues was detected by western blot analysis; ADP, AMP, ATP contents, and the energy charge were detected using Elisa; and apoptosis of gastric smooth muscle cells was detected by flow cytometry. The rat gastric smooth muscle cells were cultured in vitro, and treated with an AMPK inhibitor and an agonist. At 24 and 48 h, the effects of AMPK on apoptosis and energy metabolism of gastric smooth muscle cells were observed. Reduced spontaneous contractions, AMPK activation, cell apoptosis, and energy metabolism disorders were observed in gastric smooth muscle tissues of a diabetic rat, and AMPK activation was associated with an increased ratio of ADP/ATP, AMP/ATP, LKB1 activity, and CaMMKβ expression. From in vitro cell culture experiments, we found that AMPK activation of high-glucose conditions promoted cell apoptosis. Inhibition of AMPK had no obvious effect on apoptosis at the early stage with high glucose, but the inhibitory effect was significant at the late stage with high glucose. AMPK can regulate both mitochondrial metabolism and glycolysis pathways under high-glucose conditions. During the early stage with high glucose, AMPK was the main promotion factor of the mitochondrial metabolism pathway, but did not increase the ATP production, AMPK also promoted the glycolysis pathway. During the late stage with high glucose, AMPK was a major inhibitor of the mitochondrial pathway, and still played a role in promoting the glycolytic pathway, which acted as the main regulator. Apoptosis and energy metabolism disorders were present in gastric smooth muscle cells during the occurrence of DGP. Under high-glucose condition, AMPK was activated, which can promote apoptosis, change the energetic metabolism pathway of cells, inhibit mitochondrial energy metabolism, and promote glycolysis.









Similar content being viewed by others
Change history
04 June 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Zhao, J., Frøkjaer, J. B., Drewes, A. M., & Ejskjaer, N. (2006). Upper gastrointestinal sensory-motor dysfunction in diabetes mellitus. World Journal of Gastroenterology, 12(18), 2846–2857.
Ramzan, Z., Duffy, F., Gomez, J., Fisher, R. S., & Parkman, H. P. (2011). Continuous glucose monitoring in gastroparesis. Digestive Diseases and Sciences, 56(9), 2646–2655.
Langendorf, C. G., & Kemp, B. E. (2015). Choreography of AMPK activation. Cell Research, 25(1), 5–6.
Meng, S., Cao, J., He, Q., Xiong, L., Chang, E., Radovick, S., Wondisford, F. E., & He, L. (2015). Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. Journal of Biological Chemistry, 290(6), 3793–3802.
Oakhill, J. S., Steel, R., Chen, Z. P., Scott, J. W., Ling, N., Tam, S., & Kemp, B. E. (2011). AMPK is a direct adenylate charge-regulated protein kinase. Science, 332(6036), 1433–1435.
Garcia, D., & Shaw, R. J. (2017). AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Molecular Cell, 66(6), 789–800.
Yuan, H. D., Quan, H. Y., Zhang, Y., Kim, S. H., & Chung, S. H. (2010). 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Molecular Medicine Reports, 3(5), 825–831.
Song, W., Yan, C. Y., Zhou, Q. Q., & Zhen, L. L. (2017). Galangin potentiates human breast cancer to apoptosis induced by TRAIL through activating AMPK. Biomedicine and Pharmacotherapy, 89, 845–856.
Gui, D., Cui, Z., Zhang, L., Yu, C., Yao, D., Xu, M., Chen, M., Wu, P., Li, G., Wang, L., & Huang, X. (2017). Salidroside attenuates hypoxia-induced pulmonary arterial smooth muscle cell proliferation and apoptosis resistance by upregulating autophagy through the AMPK-mTOR-ULK1 pathway. BMC Pulmonary Medicine, 17(1), 191.
Zhang, M.-H., Jiang, J.-Z., Cai, Y.-L., Piao, L.-H., & Jin, Z. (2017). Significance of dynamic changes in gastric smooth muscle cell apoptosis, PI3K–AKT–mTOR and AMPK–mTOR signaling in a rat model of diabetic gastroparesis. Molecular Medicine Reports, 16(2), 1530–1536.
Wu, X. F., Chen, X. L., Zheng, X. N., Guo, X., Xie, Z. Q., Liu, L., Wei, X. R., & Yue, Z. H. (2018). [Effect of different stimulating strength of electroacupuncture on gastrointestinal motility and RhoA/ROCK signaling in gastric antral smooth muscle in diabetic gastroparesis rats]. Zhen Ci Yan Jiu=Acupuncture research/[Zhongguo yi xue ke xue yuan Yi xue qing bao yan jiu suo bian ji], 43(3), 169–174.
Da Silva, L. M., da Silva, R. C. M. V. A. F., Maria-Ferreira, D., Beltrame, O. C., da Silva-Santos, J. E., & Werner, M. F. P. (2017). Vitamin C improves gastroparesis in diabetic rats: effects on gastric contractile responses and oxidative stress. Digestive Diseases and Sciences, 62(9), 2338–2347.
Fang, X. S., Zhang, M. H., Guo, J. Y., Jin, Z. (2018). Effects of insulin-like growth factor-1 on endoplasmic reticulum stress and autophagy in ratgastric smooth muscle cells cultured at different glucose concentrations in vitro. Molecular and Cell Biochemistry. https://doi.org/10.1007/s11010-018-3388-7.
Dalziel, J. E., Fraser, K., Young, W., McKenzie, C. M., Bassett, S. A., & Roy, N. C. (2017). Gastroparesis and lipid metabolism-associated dysbiosis in Wistar-Kyoto rats. American Journal of Physiology-Gastrointestinal and Liver Physiology, 313(1), G62–G72.
Wilbring, M., Ebner, A., Schoenemann, K., Knaut, M., Tugtekin, S. M., Zatschler, B., Waldow, T., Alexiou, K., Matschke, K., & Deussen, A. (2013). Heparinized blood better preserves cellular energy charge and vascular functions of intraoperatively stored saphenous vein grafts in comparison to isotonic sodium-chloride-solution. Clinical Hemorheology and Microcirculation, 55(4), 445–455.
Yang, J. W., Peng, Y., Chen, H. J., Zhang, C. C., Liu, W. W., Liu, L., Liu, M., & Lin, Y. P. (2017). [Effect of electroacupuncture intervention on gastrointestinal motility and expression of insulin-like growth factor 1 and its receptor proteins in gastric antrum in diabetic gastroparesis rats]. Zhen Ci Yan Jiu= Acupuncture research/[Zhongguo yi xue ke xue yuan Yi xue qing bao yan jiu suo bian ji], 42(4), 315–320.
Xu, L., Li, Z., & Guo, F. (2013). Curcumin improves expression of ghrelin through attenuating oxidative stress in gastric tissues of streptozotocin-induced diabetic gastroparesis rats. European Journal of Pharmacology, 718(1-3), 219–225.
Cai, Y. L., Xu, D. Y., Li, X. L., Qiu, Z. X., Jin, Z., & Xu, W. X. (2009). C-type natriuretic-peptide-potentiated relaxation response of gastric smooth muscle in streptozotocin-induced diabetic rats. World Journal of Gastroenterology, 15(17), 2125–2131.
Chen, X., Fu, X. S., Li, C. P., & Zhao, H. X. (2014). ER stress and ER stress-induced apoptosis are activated in gastric SMCs in diabetic rats. World Journal of Gastroenterology, 20(25), 8260–8267.
An, X., Long, C., Deng, X., Tang, A., Xie, J., Chen, L., & Wang, Z. (2017). Higenamine inhibits apoptosis and maintains survival of gastric smooth muscle cells in diabetic gastroparesis rat model via activating the β2-AR/PI3K/AKT pathway. Biomedicine and Pharmacotherapy, 95, 1710–1717.
Liu, Z. Y., Hu, S. P., Ji, Q. R., Yang, H. B., Zhou, D. H., & Wu, F. F. (2017). Sevoflurane pretreatment inhibits the myocardial apoptosis caused by hypoxia reoxygenation through AMPK pathway: an experimental study. Asian Pacific Journal of Tropical Medicine, 10(2), 148–151.
Hardie, D. G., & Lin, S. C. (2017). AMP-activated protein kinase—not just an energy sensor. F1000Res, 6, 1724.
Kramer, P. A., Ravi, S., Chacko, B., Johnson, M. S., & Darley-Usmar, V. M. (2014). A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biology, 2, 206–210.
Funding
This study was financially supported by grants of the National Natural Science Foundation of China (No. 81560142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Mh., Fang, Xs., Guo, Jy. et al. Effects of AMPK on Apoptosis and Energy Metabolism of Gastric Smooth Muscle Cells in Rats with Diabetic Gastroparesis. Cell Biochem Biophys 77, 165–177 (2019). https://doi.org/10.1007/s12013-019-00870-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-019-00870-9