Abstract
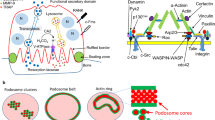
Osteoclast is the only cell that can degrade bone tissue in vivo. Recent studies have shown the important role of cytoskeleton dynamics in osteolysis and the formation of podosome belt in osteoclasts. This process is regulated by the dynamic microtubule (MT) network. We treated osteoclast precursor cells Raw264.7 with low concentration of nocodazole (10 nM) and antineoplastic drug taxol (10 nM) to block MT turnover, and used end binding protein 1 fused to GFP to track the movement of microtubules in induced osteoclasts. We show that low concentrations of nocodazole and taxol interfere with the formation of podosome belt, and reduce TRAP activity of induced osteoclasts. These results suggest that the effect of taxol on MT dynamics may be used clinically to reduce osteoclast activity and potentially prevent development of osteoporosis and other metabolic bone diseases.





Similar content being viewed by others
References
Gravallese, E. M., Harada, Y., Wang, J. T., et al. (1998). Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. American Journal of Pathology, 152, 943–951.
Linder, S., & Aepfelbacher, M. (2003). Podosomes: adhesion hot-spots of invasive cells. Trends in Cell Biology, 13, 376–385.
Georgess, D., Machuca-Gayet, I., Blangy, A., & Jurdic, P. (2014). Podosome organization drives osteoclast-mediated bone resorption. Cell adhesion & migration, 8(3), 1–13.
Saltel, F., Daubon, T., Juin, A., Ganuza, I. E., Veillat, V., et al. (2011). Invadosomes: Intriguing structures with promise. European Journal of Cell Biology, 90, 100–107.
Destaing, O., Saltel, F., Geminard, J. C., Jurdic, P., & Bard, F. (2003). Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Molecular Biology of the Cell, 14, 407–416.
Jurdic, P., Saltel, F., Chabadel, A., & Destaing, O. (2006). Podosome and sealing zone: specificity of the osteoclast model. European Journal of Cell Biology, 85, 195–202.
Okumura, S., Mizoguchi, T., Sato, N., Yamaki, M., Kobayashi, Y., Yamauchi, H., et al. (2006). Coordination of microtubules and the actin cytoskeleton is important in osteoclast function, but calcitonin disrupts sealing zones without affecting microtubule networks. Bone, 39(4), 684–693.
Destaing, O., Saltel, F., Gilquin, B., Chabadel, A., Khochbin, S., Ory, S., et al. (2005). A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. Journal of Cell Science, 118, 2901–2911.
Gil-Henn, H., Destaing, O., & Sims, N. A. (2007). Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2 −/−mice. Journal of Cell Biology, 178(6), 1053–1064.
Akisaka, T., Yoshida, H., Suzuki, R., & Takama, K. (2008). Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture. Cell and Tissue Research, 331, 625–641.
Akisaka, T., Yoshida, H., & Takigawa, T. (2011). Differential distribution of posttranslationally modified microtubules in osteoclasts. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society, 59, 630–638.
Kopp, P., Lammers, R., Aepfelbacher, M., Woehlke, G., Rudel, T., Machuy, N., et al. (2006). The kinesin KIF1C and microtubule plus ends regulate podosome dynamics in macrophages. Molecular biology of the cell, 17, 2811–2823.
Akhmanova, A., & Hoogenraad, C. C. (2005). Microtubule plus-endtracking proteins: mechanisms and functions. Current Opinion in Cell Biology, 17, 47–54.
Vaughan, K. T. (2005). TIP maker and TIP marker; EB1 as a master controller of microtubules plus ends. Journal of Cell Biology, 171, 197–200.
Mimori-Kiyosue, Y., Shiina, N., and Tsukita, S. (2000). Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. The Journal of cell biology. 148:505–518.
Abu-Amer, Y., Ross, F. P., Schlesinger, P., Tondravi, M. M., & Teitelbaum, S. L. (1997). Substrate recognition by osteoclast precursors inducesC-src/microtubule association. The Journal of cell biology, 137, 247–258.
Ng, P. Y., Cheng, T. S., Zhao, H., Ye, S., Sm Ang, E., Khor, E. C., et al. (2013). Disruption of the dynein-dynactin complex unveils motor-specific functions in osteoclast formation and bone resorption. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 28, 119–134.
Ye, S., Fowler, T. W., Pavlos, N. J., Ng, P. Y., Liang, K., Feng, Y., et al. (2011). LIS1 regulates osteoclast formation and function through its interactions with dynein/dynactin and Plekhm1. PLoS ONE, 6, e27285.
Pavlos, N. J., Cheng, T. S., Qin, A., Ng, P. Y., Feng, H. T., Ang, E. S., et al. (2011). Tctex-1, a novel interaction partner of Rab3D, is required for osteoclastic bone resorption. Molecular and Cellular Biology, 31, 1551–1564.
Hazama, R., Qu, X., Yokoyama, K., Tanaka, C., Kinoshita, E., He, J., et al. (2009). ATP-induced osteoclast function: the formation of sealing-zone like structure and the secretion of lyticgranules via microtubule-deacetylation under the control of Syk. Genes to cells : devoted to molecular & cellular mechanisms, 14, 871–884. 813.
Stenbeck, G., & Horton, M. A. (2004). Endocytic trafficking in actively resorbing osteoclasts. Journal of Cell Science, 117, 827–836.
Albiges-Rizo, C., Destaing, O., Fourcade, B., Planus, E., & Block, M. R. (2009). Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. Journal of Cell Science, 122, 3037–3049.
Murphy, D. A., & Courtneidge, S. A. (2011). The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nature Reviews Molecular Cell Biology, 12, 413–426.
Huang, S., Eleniste, P.P., Wayakanon, K. (2014). The Rho-GEF Kalirin regulates bone mass and the function of osteoblasts and osteoclasts. Bone. 60:235–245
Hong, J. M., Teitelbaum, S. L., Kim, T. H., Ross, F. P., Kim, S. Y., & Kim, H. J. (2011). Calpain-6, a target molecule of glucocorticoids, regulates osteoclastic bone resorption via cytoskeletal organization and microtubule acetylation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research., 26, 657–665.
McMichael, B. K., Cheney, R. E., & Lee, B. S. (2010). Myosin X regulates sealing zone patterning in osteoclasts through linkage of podosomes and microtubules. The Journal of biological chemistry, 285, 9506–9515.
Matsumoto, T., Nagase, Y., Hirose, J., Tokuyama, N., Yasui, T., Kadono, Y., Ueki, K., Kadowaki, T., Nakamura, K., Tanaka, S. (2013). Regulation of bone-resorption and sealing zone formation in osteoclasts occurs through Akt-mediated microtubule stabilization. Journal of Bone and Mineral Research. 28(5):1191–1202.
Duellberg, C., Fourniol, F. J., Maurer, S. P., Roostalu, J., & Surrey, T. (2013). End-binding proteins and Ase1/PRC1 define local functionality of structurally distinct parts of the microtubule cytoskeleton. Trends in Cell Biology, 23, 54–63.
Evans, J. G., et al. (2003). Macrophage podosomes assemble at the leading lamella by growth and fragmentation. Journal of Cell Biology, 161, 697–705.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yunfan Ti and Lingjun Zhou have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Ti, Y., Zhou, L., Wang, R. et al. Inhibition of Microtubule Dynamics Affects Podosome Belt Formation During Osteoclast Induction. Cell Biochem Biophys 71, 741–747 (2015). https://doi.org/10.1007/s12013-014-0258-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0258-0