Abstract
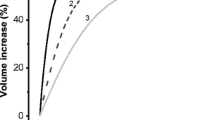
Using a microfluidic volume sensor, we studied the dynamic effects of Hg2+ on hypotonic stress-induced volume changes in CHO cells. A hypotonic challenge to control cells caused them to swell but did not evoke a significant regulatory volume decrease (RVD). Treatment with 100 μM HgCl2 caused a substantial increase in the steady-state volume following osmotic stress. Continuous hypotonic challenge following a single 10-min exposure to HgCl2 produced a biphasic volume increase with a steady-state volume 100% larger than control cells. Repeated hypotonic challenges to cells exposed once to Hg2+ resulted in a sequential approach to the same steady-state volume. Stimulation after reaching steady state caused a reduction in peak cell volume. Repeated stimulation was different than continuous stimulation resulting in a more rapid approach to steady state. Substituting extracellular Na+ with impermeant NMDG+ in the hypotonic solution produced a rapid RVD-like volume decrease and eliminated the Hg2+-induced excess swelling. The volume decrease in the presence of Hg2+ was inhibited by tetraethylammonium and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium, blockers of K+ and Cl− channels, respectively, suggesting that part of the Hg2+ effect was increasing NaCl influx over KCl efflux. The presence of multiple phases of steady-state volume and their sensitivity to the stimulation history suggests that factors beyond solute fluxes, such as modification of mechanical stress within the cytoskeleton also plays a role in the response to hypotonic stress.










Similar content being viewed by others
References
Atchison, W. D., & Hare, M. F. (1994). Mechanisms of methylmercury-induced neurotoxicity. FASEB Journal, 8, 622–629.
Zalups, R. K. (2000). Molecular interactions with mercury in the kidney. Pharmacological Review, 52, 113–143.
Aschner, M., Vitarella, D., Allen, J. W., Conklin, D. R., & Cowan, K. S. (1998). Methylmercury-induced inhibition of regulatory volume decrease in astrocytes: Characterization of osmoregulator efflux and its reversal by amiloride. Brain Research, 811, 133–142.
Ballatori, N., & Boyer, J. L. (1996). Disruption of cell volume regulation of mercuric chloride is mediated by an increase in sodium permeability and inhibition of an osmolyte channel in skate hepatocytes. Toxicology and Applied Pharmacology, 140, 404–410.
Ballatori, N., Shi, C., & Boyer, J. L. (1988). Altered plasma membrane ion permeability in mercury-induced cell injury: Studies in heptatocytes of elasmobranch Raja erinacea. Toxicology and Applied Pharmacology, 95, 279–291.
Rothstein, A., & Mack, E. (1991). Actions of mercurials on cell volume regulation of dissociated MDCK cells. American Journal of Physiology. Cell Physiology, 260, C113–C121.
Aschner, M., Eberle, N. B., Miller, K., & Kimelberg, H. K. (1990). Interactions of methylmercury with rat primary astrocyte cultures: Inhibition of rubidium and glutamate uptake and induction of swelling. Brain Research, 530, 245–250.
Albrecht, J., Talbot, M., Kimelberg, H. K., & Aschner, M. (1993). The role of sulfhydryl groups and calcium in the mercuric chloride-induced inhibition of glutamate uptake in rat primary astrocyte cultures. Brain Research, 607, 249–254.
Yasui, M., Hazama, A., Kwon, T.-H., Nielsen, S., Guggino, W. B., & Agre, P. (1999). Rapid gating and anion permeability of an intracellular aquaporin. Nature, 402, 184–187.
Barone, L. M., Shih, C., & Wasserman, B. P. (1997). Mercury-induced conformational changes and identification of conserved surface loops in plasma membrane aquaporins from higher plants: Topology of PMIP31 from Beta vulgaris L. Journal of Biological Chemistry, 272, 30672–30677.
Hazama, A., Kozono, D., Guggino, W. B., Agre, P., & Yasui, M. (2002). Ion permeation of AQP6 water channel protein. Journal of Biological Chemistry, 277, 29224–29230.
Chavez, E., & Holguin, J. A. (1988). Mitochondrial calcium release as induced by Hg2+. Journal of Biological Chemistry, 263, 3582–3587.
Chavez, E., Zazueta, C., Osornio, A., Holguin, J. A., & Miranda, M. E. (1991). Protective behavior of captopril on Hg2+-induced toxicity on kidney mitochondria: In vivo and in vitro experiments. Journal of Pharmacological and Experimental Therapeutics, 256, 385–390.
Lund, B.-O., Miller, D. M., & Woods, J. S. (1993). Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochemical Pharmacology, 45, 2017–2024.
Weinberg, J. M., Harding, P. G., & Humes, H. D. (1982). Mitochondrial bioenergetics during the initiation of mercuric chloride-induced renal injury: I. Direct effects of in vitro mercuric chloride on renal mitochondrial function. Journal of Biological Chemistry, 257, 60–67.
Anner, B. M., Moosmayer, M., & Imesch, E. (1993). Chelation of mercury by ouabain-sensitive and ouabain-resistant renal Na+/K+-ATPase. Biochemical and Biophysics Research Communications, 167, 1115–1121.
Anner, B. M., Moosmayer, M., & Imesch, E. (1992). Mercury blocks Na-K-ATPase by a ligand-dependent and reversible mechanism. American Journal of Physiology. Renal Physiology, 262, F830–F836.
Stoiber, T., Degen, G. H., Bolt, H. M., & Unger, E. (2004). Interaction of mercury (II) with the microtubule cytoskeleton in IMR-32 neuroblastoma cells. Toxicology Letters, 151, 99–104.
Mel, H. C., & Reed, T. A. (1981). Biophysical responses of red cell-membrane systems to very low concentrations of inorganic mercury. Cell Biophysics, 3, 233–250.
Jensen, B. S., Kramhoeft, B., Jessen, F., Lambert, I. H., & Hoffmann, E. K. (1993). HgCl2-induced ion transport pathways in Ehrlich ascites tumor cells. Cellular Physiology and Biochemistry, 3, 97–110.
Vitarella, D., Kimelberg, H. K., & Aschner, M. (1996). Inhibition of regulatory volume decrease in swollen rat primary astrocyte cultures by methylmercury is due to increased amiloride-sensitive Na+ uptake. Brain Research, 732, 169–178.
Kramhoft, B., Lambert, I. H., & Pedersen, R. (1989). Mercuric chloride activates latent, anion-dependent cation transport systems in the plasma membrane of Ehrlich ascites tumor cells. Pharmacology and Toxicology, 64, 421–425.
Elliget, K. A., Phelps, P. C., & Trump, B. F. (1991). Mercury(II) chloride-induced alteration of actin filaments in cultured primary rat proximal tubule epithelial cells. Cell Biology and Toxicology, 7, 263–280.
Ateya, D. A., Sachs, F., Gottlieb, P. A., Besch, S., & Hua, S. Z. (2005). Volume cytometry: Microfluidic sensor for high-throughput screening in real time. Analytical Chemistry, 77, 1290–1294.
Garnovskaya, M. N., Mukhin, Y. V., Vlasova, T. M., & Raymond, J. R. (2003). Hypertonicity activates Na+/H+ exchange through Janus kinase 2 and Calmodulin. Journal of Biological Chemistry, 278, 16908–16915.
Qian, N.-X., Pastor-Anglada, M., & Englesberg, E. (1991). Evidence for coordinating regulation of the A system for amino acid transport and the mRNA for the a1 subunit of the Na+, K+-ATPase gene in Chinese hamster ovary cells. Proceedings of National Academy of Sciences of the USA, 88, 3416–3420.
Tonini, R., Ferroni, A., Valenzuela, S. M., Warton, K., Campbell, T. J., Breit, S. N., & Mazzanti, M. (2000). Functional characterization of the NCC27 nuclear protein in stable transfected CHO-K1 cells. FASEB Journal, 14, 1171–1178.
Gabriel, S. E., Price, E. M., Boucher, R. C., & Stutts, M. J. (1992). Small linear chloride channels are endogenous to nonepithelial cells. American Journal of Physiology. Cell Physiology, 263, C708–C713.
Lalik, P., Krafte, D. S., Volberg, W. A., & Ciccarelli, R. B. (1993). Characterization of endogeneous sodium channel gene expressed in Chinese hamster ovary cells. American Journal of Physiology. Cell Physiology, 264, C803–C809.
Skryma, R., Prevarskaya, N., Vacher, P., & Dufy, B. (1994). Voltage-dependent Ca2+ channels in Chinese hamser ovary (CHO) cells. FEBS Letters, 349, 289–294.
Alvarez-Leefmans, F. J., Herrera-Perez, J. J., Marquez, M. S., & Blanco, V. M. (2006). Simultaneous measurement of water volume and pH in single cells using BCECF and fluorescence imaging microscopy. Biophysical Journal, 90, 608–618.
Borgnia, M., Nielsen, S., Engel, A., & Agre, P. (1999). Cellular and molecular biology of the aquaporin water channels. Annual Review of Biochemistry, 68, 425–428.
Golgelein, H. (1988). Chloride channels in epithelia. Biochimica et Biophysica Acta, 947, 521–547.
Ikeda, S. R., & Korn, S. J. (1995). Influence of permeating ions on potassium channel block by external tetraethylammonium. Journal of Physiology, 486, 267–272.
Pourahmad, J., & O’Brien, P. J. (2003). Involvement of intracellular Na+ accumulation in Hg2+ or Cd2+ induced cytotoxicity. Journal de Physique IV: Proceedings, 107, 1095–1098.
Vazquez, N., Monroy, A., Dorantes, E., Munoz-Clares, R. A., & Gamba, G. (2002). Functional differences between flounder and rat thazide-sensitive Na-Cl cotransporter. American Journal of Physiology. Renal Physiology, 282, F599–F607.
Jacoby, S. C., Gagnon, E., Caron, L., Chang, J., & Isenring, P. (1999). Inhibition of Na+-K+-2Cl- cotransport by mercury. American Journal of Physiology. Cell Physiology, 277, C684–C692.
Anner, B. M., & Moosmayer, M. (1992). Mercury inhibits Na-K-ATPase primarily at the cytoplasmic side. American Journal of Physiology. Renal Physiology, 262, F843–F848.
Benos, D. J. (1982). Amiloride: A molecular probe of sodium transport in tissues and cells. American Journal of Physiology. Cell Physiology, 242, C131–C145.
Guo, T. L., Miller, M. A., Shapiro, I. M., & Shenker, B. J. (1998). Mercuric chloride induces apoptosis in human T lymphocytes: Evidence of mitochondrial dysfunction. Toxicology and Applied Pharmacology, 153, 250–257.
Livine, A., & Hoffmann, E. K. (1990). Cytoplasmic acidification and activation of Na+/H+ exchange during regulatroy volume decrease in Ehrlich ascites tumor cells. Journal of Membrane Biology, 114, 153–157.
Gutknecht, J. (1981). Inorganic mercury (Hg2+) transport through lipid bilayer membranes. Journal of Membrane Biology, 61, 61–66.
Jacobson, M. D., Weil, M., & Raff, M. C. (1996). Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. Journal of Cell Biology, 133, 1041–1051.
Becker, D., Blase, C., Bereiter-Hahn, J., & Jendrach, M. (2005). TRPV4 exhibits a functional role in cell-volume regulation. Journal of Cell Science, 118, 2435–2440.
Rothstein, A., & Mack, E. (1992). Volume-activated calcium uptake: its role in cell volume regulation of Madin-Darby canine kidney cells. American Journal of Physiology. Cell Physiology., 262, C339–C347.
Charras, G. T., Hu, C. K., Coughlin, M., & Mitchison, T. J. (2006). Reassembly of contractile actin cortex in cell blebs. Journal of Cell Biology, 175, 477–490.
Charras, G. T., Yarrow, J. C., Horton, M. A., Mahadevan, L., & Mitchison, T. J. (2005). Non-equilibration of hydrostatic pressure in blebbing cells. Nature, 435, 365–369.
Bonacker, D., Stoiber, T., Wang, M., Bohm, K., Prots, I., Unger, E., Thier, R., Bolt, H., & Degen, G. (2004). Genotoxicity of inorganic mercury salts based on disturbed microtubule function. Archives of Toxicology, 78, 575–583.
Sheetz, M. P., Sable, J. E., & Dobereiner, H.-G. (2006). Continuous membrane-cytoskeleton adhesion requires continuous accomodation to lipid and cytoskeleton dynamics. Annual Review of Biophysics and Bimolecular Structure, 35, 417–434.
Ziyadeh, F. N., Mills, J. W., & Kleinzeller, A. (1992). Hypotonicity and cell volume regulation in shark rectal gland: role of organic osmolytes and F-actin. American Journal of Physiology. Renal Physiology., 262, F468–F479.
Zucker, R., Elstein, K., Easterling, R., Ting-Beall, H., Allis, J., & Massaro, E. (1988). Effects of tributyltin on biomembranes: Alteration of flow cytometric parameters and inhibition of Na+, K+-ATPase two-dimensional crystallization. Toxicology and Applied Pharmacology, 96, 393–403.
Heinrich, V., & Rawicz, W. (2005). Automated, high-resolution micropipet aspiration reveals new insight into the physical properties of fluid membranes. Langmuir, 21, 1962–1971.
Akinlaja, J., & Sachs, F. (1998). The breakdown of cell membranes by electrical and mechanical stress. Biophysics Journal, 75, 247–254.
Bloom, M., Evans, E., & Mouritsen, O. G. (1991). Physical properties of the fluid lipid-bilayer component of cell membranes : A perspective. Quarterly Reviews of Biophysics, 24, 293–397.
Venosa, R. A. (2003). Hypotonic stimulation of the Na+ active transport in frog skeletal muscle: Role of the cytoskeleton. Journal of Physiology, 548, 451–459.
Guilak, F., Erickson, G. R., & Ting-Beall, H. P. (2002). The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophysics Journal, 82, 720–727.
Wan, X., Harris, J. A., & Morris, C. E. (1995). Responses of neurons to extreme osmomechanical stress. Journal of Membrane Biology, 145, 21–31.
Acknowledgments
This work is supported by National Institute of Health Grant DK77302, NHLBI (FS) and by New York State Office of Science, Technology & Academic Research (NYSTAR).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
M-I. CHO cells in isotonic solution. The image was taken at 1 min/frame for 40 min (AVI 3041 kb)
M-II. CHO cells in hypotonic solution containing 100 μM HgCl2. The image was taken at 1 min/frame for 40 min (AVI 2654 kb)
Rights and permissions
About this article
Cite this article
Heo, J., Meng, F., Sachs, F. et al. Dynamic Effects of Hg2+-induced Changes in Cell Volume. Cell Biochem Biophys 51, 21–32 (2008). https://doi.org/10.1007/s12013-008-9010-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-008-9010-y