Abstract
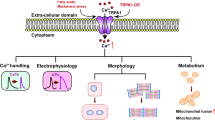
Hyperglycaemia, a key metabolic abnormality in diabetes mellitus, is implicated in pathological cardiogenesis during embryological development. However, the underlying mechanisms and potential therapeutic targets remain unknown. We, therefore, studied the effect of hyperglycaemia on mouse embryonic stem cell (mESC) cardiac differentiation. The mESCs were differentiated via embryoid body (EB) formation and cultured under conditions with baseline (25 mM) or high (50 mM) glucose. Time-lapse microscopy images of pulsatile mESCs and Ca2+ transients were recorded. Biomarkers of cellular changes were detected using immunocytochemistry, terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) assay, and Western blot analyses. Differentiated, spontaneously beating mESCs stained positive for cardiac troponin T, α-actinin 2, myosin heavy chain, and connexin 43. Hyperglycaemia decreased the EB diameter and number of beating EBs as well as the cellular amplitude of contraction, the Ca2+ transient, and the contractile response to caffeine (1 mM), but had no effect on the expression of the sarco-endoplasmic reticulum calcium transport ATPase 2 (SERCA 2). Furthermore, hyperglycaemia decreased the expression of B cell lymphoma 2 (Bcl-2) and increased the expression of cytoplasmic cytochrome c and the number of TUNEL-positive cells, but had no effect on the expression of one of the mitochondrial fusion regulatory proteins, optic atrophy protein 1 (OPA1). Overall, hyperglycaemia suppressed the mESC cardiomyocyte-like differentiation and induced contractile dysfunction. The results are consistent with mechanisms involving abnormal Ca2+ handling and mitochondrial-dependent apoptosis, factors which represent potential therapeutic targets in developmental diabetic cardiac disease.

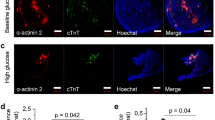
taken from undifferentiated mESCs. Inset: Negative (-ve) control in which the primary antibody was omitted. Arrowheads indicate nuclei of inactivated mouse embryonic fibroblasts (iMEFs) in the feeder layer. Scale bar = 50 µm. n = 3 replicates. c‒e Representative confocal microscopy images of βIII-tubulin, α-smooth muscle actin (α-SMA), α1-fetoprotein, Hoechst, and merged images. Insets: Negative (-ve) controls in which the primary antibodies were omitted. Scale bar = 10 µm. n = 3 replicates for each germ layer









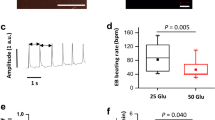
taken from actively beating areas of embryoid bodies. Scale bar = 5 µm. c Quantitative analysis of the cellular Mitotracker Green fluorescence intensity (n = 4‒6 replicates per group). Data are shown as box plot and the mean (filled square). Glu, glucose
Similar content being viewed by others
Data Availability
Data and material available upon request.
References
Aberg, A., Westbom, L., & Kallen, B. (2001). Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Human Development, 61, 85–95
Tinker, S. C., Gilboa, S. M., Moore, C. A., Waller, D. K., Simeone, R. M., Kim, S. Y., et al. (2020). Specific birth defects in pregnancies of women with diabetes: national birth defects prevention study, 1997–2011. American Journal of Obstetrics and Gynecology, 222, 176.e1-176.e11
Loffredo, C. A., Wilson, P. D., & Ferencz, C. (2001). Maternal diabetes: an independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology, 64, 98–106
Floris, I., Descamps, B., Vardeu, A., Mitic, T., Posadino, A. M., Shantikumar, S., et al. (2015). Gestational diabetes mellitus impairs fetal endothelial cell functions through a mechanism involving microRNA-101 and histone methyltransferase enhancer of zester homolog-2. Arteriosclerosis, Thrombosis, and Vascular Biology, 35, 664–674
Kumar, S. D., Dheen, S. T., & Tay, S. S. (2007). Maternal diabetes induces congenital heart defects in mice by altering the expression of genes involved in cardiovascular development. Cardiovascular Diabetology, 6, 34
Reinking, B. E., Wedemeyer, E. W., Weiss, R. M., Segar, J. L., & Scholz, T. D. (2009). Cardiomyopathy in offspring of diabetic rats is associated with activation of the MAPK and apoptotic pathways. Cardiovascular Diabetology, 8, 43
Basu, M., & Garg, V. (2018). Maternal hyperglycemia and fetal cardiac development: clinical impact and underlying mechanisms. Birth Defects Research, 110, 1504–1516
Narchi, H., & Kulaylat, N. (2000). Heart disease in infants of diabetic mothers. Images in Paediatric Cardiology, 2, 17–23
Steinhauser, M. L., & Lee, R. T. (2011). Regeneration of the heart. EMBO Molecular Medicine, 3, 701–712
Nag, A. C., Lee, M. L., & Sarkar, F. H. (1996). Remodelling of adult cardiac muscle cells in culture: dynamic process of disorganization and reorganization of myofibrils. Journal of Muscle Research and Cell Motility, 17, 313–334
Wobus, A. M., Guan, K., Yang, H. T., & Boheler, K. R. (2002). Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods in Molecular Biology, 185, 127–156
Shi, M., Tien, N. T., de Haan, L., Louisse, J., Rietjens, I., & Bouwmeester, H. (2020). Evaluation of in vitro models of stem cell-derived cardiomyocytes to screen for potential cardiotoxicity of chemicals. Toxicology In Vitro, 67, 104891
Wang, X., & Yang, P. (2008). In vitro differentiation of mouse embryonic stem (mES) cells using the hanging drop method. Journal of Visualized Experiments. https://doi.org/10.3791/825
Lee, M. Y., Cagavi Bozkulak, E., Schliffke, S., Amos, P. J., Ren, Y., Ge, X., et al. (2011). High density cultures of embryoid bodies enhanced cardiac differentiation of murine embryonic stem cells. Biochemical and Biophysical Research Communications, 416, 51–57
Ali, N. N., Xu, X., Brito-Martins, M., Poole-Wilson, P. A., Harding, S. E., & Fuller, S. J. (2004). Beta-adrenoceptor subtype dependence of chronotropy in mouse embryonic stem cell-derived cardiomyocytes. Basic Research in Cardiology, 99, 382–391
Guan, K., Furst, D. O., & Wobus, A. M. (1999). Modulation of sarcomere organization during embryonic stem cell-derived cardiomyocyte differentiation. European Journal of Cell Biology, 78, 813–823
Crespo, F. L., Sobrado, V. R., Gomez, L., Cervera, A. M., & McCreath, K. J. (2010). Mitochondrial reactive oxygen species mediate cardiomyocyte formation from embryonic stem cells in high glucose. Stem Cells, 28, 1132–1142
Han, S. S., Wang, G., Jin, Y., Ma, Z. L., Jia, W. J., Wu, X., et al. (2015). Investigating the mechanism of hyperglycemia-induced fetal cardiac hypertrophy. PLoS ONE, 10, e0139141
Xu, X., Ruan, L., Tian, X., Pan, F., Yang, C., & Liu, G. (2020). Calcium inhibitor inhibits high glucoseinduced hypertrophy of H9C2 cells. Molecular Medicine Reports, 22, 1783–1792
Yang, P., Chen, X., Kaushal, S., Reece, E. A., & Yang, P. (2016). High glucose suppresses embryonic stem cell differentiation into cardiomyocytes : High glucose inhibits ES cell cardiogenesis. Stem Cell Research & Therapy, 7, 187
Grune, T., Ott, C., Haseli, S., Hohn, A., & Jung, T. (2019). The “MYOCYTER” - Convert cellular and cardiac contractions into numbers with ImageJ. Scientific Reports, 9, 15112
Francis, M., Waldrup, J., Qian, X., & Taylor, M. S. (2014). Automated analysis of dynamic Ca2+ signals in image sequences. Journal of Visualized Experiments. https://doi.org/10.3791/51560
Aboalgasm, H., Petersen, M., & Gwanyanya, A. (2020). Improvement of cardiac ventricular function by magnesium treatment in chronic streptozotocin-induced diabetic rat heart. Cardiovascular Journal of Africa, 31, 1–8
Nichols, J., Zevnik, B., Anastassiadis, K., Niwa, H., Klewe-Nebenius, D., Chambers, I., et al. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell, 95, 379–391
Chambers, I., Colby, D., Robertson, M., Nichols, J., Lee, S., Tweedie, S., et al. (2003). Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell, 113, 643–655
Chen, L., Gong, Q., Stice, J. P., & Knowlton, A. A. (2009). Mitochondrial OPA1, apoptosis, and heart failure. Cardiovascular Research, 84, 91–99
Sutton-McDowall, M. L., Gilchrist, R. B., & Thompson, J. G. (2010). The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction, 139, 685–695
Mochizuki, H., Ohnuki, Y., & Kurosawa, H. (2011). Effect of glucose concentration during embryoid body (EB) formation from mouse embryonic stem cells on EB growth and cell differentiation. Journal of Bioscience and Bioengineering, 111, 92–97
Nakano, H., Minami, I., Braas, D., Pappoe, H., Wu, X., Sagadevan, A., et al. (2017). Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. eLife, 6, e29330
McKee, T. J., & Komarova, S. V. (2017). Is it time to reinvent basic cell culture medium? American Journal of Physiology Cell Physiology, 312, C624–C626
Kolwicz, S. C., Jr., & Tian, R. (2011). Glucose metabolism and cardiac hypertrophy. Cardiovascular Research, 90, 194–201
Hou, Q., Lei, M., Hu, K., & Wang, M. (2015). The effects of high glucose levels on reactive oxygen species-induced apoptosis and involved signaling in human vascular endothelial cells. Cardiovascular Toxicology, 15, 140–146
Frezza, C., Cipolat, S., Martins de Brito, O., Micaroni, M., Beznoussenko, G. V., Rudka, T., et al. (2006). OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell, 126, 177–189
Lu, S., Liao, Z., Lu, X., Katschinski, D. M., Mercola, M., Chen, J., et al. (2020). Hyperglycemia acutely increases cytosolic reactive oxygen species via o-linked glcnacylation and camkii activation in mouse ventricular myocytes. Circulation Research, 126, e80–e96
Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F., & Nogueira-Machado, J. A. (2018). Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death & Disease, 9, 119
Kumar, S., Kain, V., & Sitasawad, S. L. (2012). High glucose-induced Ca2+ overload and oxidative stress contribute to apoptosis of cardiac cells through mitochondrial dependent and independent pathways. Biochimica et Biophysica Acta - General Subjects, 1820, 907–920
Choi, K. M., Zhong, Y., Hoit, B. D., Grupp, I. L., Hahn, H., Dilly, K. W., et al. (2002). Defective intracellular Ca2+ signaling contributes to cardiomyopathy in Type 1 diabetic rats. American Journal of Physiology Heart and Circulatory Physiology, 283, H1398-1408
Zhang, L., Cannell, M. B., Phillips, A. R., Cooper, G. J., & Ward, M. L. (2008). Altered calcium homeostasis does not explain the contractile deficit of diabetic cardiomyopathy. Diabetes, 57, 2158–2166
Bode, E. F., Briston, S. J., Overend, C. L., O’Neill, S. C., Trafford, A. W., & Eisner, D. A. (2011). Changes of SERCA activity have only modest effects on sarcoplasmic reticulum Ca2+ content in rat ventricular myocytes. The Journal of Physiology, 589, 4723–4729
Rosen, M. R., Myerburg, R. J., Francis, D. P., Cole, G. D., & Marban, E. (2014). Translating stem cell research to cardiac disease therapies: pitfalls and prospects for improvement. Journal of the American College of Cardiology, 64, 922–937
Kowalski, M. P., Yoder, A., Liu, L., & Pajak, L. (2012). Controlling embryonic stem cell growth and differentiation by automation: enhanced and more reliable differentiation for drug discovery. Journal of Biomolecular Screening, 17, 1171–1179
Zhou, P., & Pu, W. T. (2016). Recounting cardiac cellular composition. Circulation Research, 118, 368–370
Acknowledgements
The authors thank Desiree Bowers for tissue-culture technical assistance and Susan Cooper for technical assistance in the HUB Confocal and Light Microscope Imaging Facility.
Funding
The study was funded by the South African Medical Research Council (MRC Grant Number 29841).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Jianyong Ma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aboalgasm, H., Ballo, R., Mkatazo, T. et al. Hyperglycaemia-Induced Contractile Dysfunction and Apoptosis in Cardiomyocyte-Like Pulsatile Cells Derived from Mouse Embryonic Stem Cells. Cardiovasc Toxicol 21, 695–709 (2021). https://doi.org/10.1007/s12012-021-09660-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09660-3