Abstract
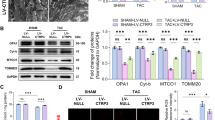
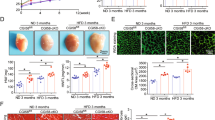
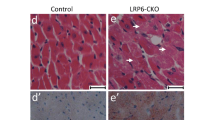
Fatty acid-binding protein 5 (FABP5) is an important member of the FABP family and plays a vital role in the metabolism of fatty acids. However, few studies have examined the role of FABP5 in pathological cardiac remodeling and heart failure. The aim of this study was to explore the role of FABP5 in transverse aortic constriction (TAC)-induced pathological cardiac remodeling and dysfunction in mice. Quantitative RT-PCR (qRT-PCR) and western blotting (WB) analysis showed that the levels of FABP5 mRNA and protein, respectively, were upregulated in hearts of the TAC model. Ten weeks after TAC in FABP5 knockout and wild type control mice, echocardiography, histopathology, qRT-PCR, and WB demonstrated that FABP5 deficiency aggravated cardiac injury (both cardiac hypertrophy and fibrosis) and dysfunction. In addition, transmission electron microscopy, ATP detection, and WB revealed that TAC caused severe impairment to mitochondria in the hearts of FABP5-deficient mice compared with that in control mice. When FABP5 was downregulated by siRNA in primary mouse cardiac fibroblasts, FABP5 silencing increased oxidative stress, reduced mitochondrial respiration, and increased the expression of myofibroblast activation marker genes in response to treatment with transforming growth factor-β. Our findings demonstrate that FABP5 deficiency aggravates cardiac pathological remodeling and dysfunction by damaging cardiac mitochondrial function.






Similar content being viewed by others
References
Dunlay, S. M., Roger, V. L., & Redfield, M. M. (2017). Epidemiology of heart failure with preserved ejection fraction. Nature Reviews: Cardiology, 14(10), 591–602. https://doi.org/10.1038/nrcardio.2017.65
Mollace, V., Rosano, G. M. C., Anker, S. D., Coats, A. J. S., Seferovic, P., Mollace, R., Tavernese, A., Gliozzi, M., Musolino, V., Carresi, C., Maiuolo, J., Macrì, R., Bosco, F., Chiocchi, M., Romeo, F., Metra, M., & Volterrani, M. (2021). Pathophysiological basis for nutraceutical supplementation in heart failure: A comprehensive review. Nutrients, 13(1), 257. https://doi.org/10.3390/nu13010257
Schirone, L., Forte, M., Palmerio, S., Yee, D., Nocella, C., Angelini, F., Pagano, F., Schiavon, S., Bordin, A., Carrizzo, A., Vecchione, C., Valenti, V., Chimenti, I., De Falco, E., Sciarretta, S., & Frati, G. (2017). A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxidative Medicine and Cellular Longevity, 2017, 1–16. https://doi.org/10.1155/2017/3920195
Burchfield, J. S., Xie, M., & Hill, J. A. (2013). Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation, 128(4), 388–400. https://doi.org/10.1161/circulationaha.113.001878
Tanai, E., & Frantz, S. (2015). Pathophysiology of heart failure. Comprehensive Physiology, 6(1), 187–214. https://doi.org/10.1002/cphy.c140055
Kehat, I., & Molkentin, J. D. (2010). Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation, 122(25), 2727–2735. https://doi.org/10.1161/circulationaha.110.942268
Bertero, E., & Maack, C. (2018). Metabolic remodelling in heart failure. Nature Reviews: Cardiology, 15(8), 457–470. https://doi.org/10.1038/s41569-018-0044-6
Doenst, T., Nguyen, T. D., & Abel, E. D. (2013). Cardiac metabolism in heart failure: implications beyond ATP production. Circulation Research, 113(6), 709–724. https://doi.org/10.1161/circresaha.113.300376
Taegtmeyer, H., Young, M. E., Lopaschuk, G. D., Abel, E. D., Brunengraber, H., Darley-Usmar, V., Des Rosiers, C., Gerszten, R., Glatz, J. F., Griffin, J. L., Gropler, R. J., Holzhuetter, H. G., Kizer, J. R., Lewandowski, E. D., Malloy, C. R., Neubauer, S., Peterson, L. R., Portman, M. A., Recchia, F. A., … Wang, T. J. (2016). Assessing cardiac metabolism: A scientific statement from the american heart association. Circulation Research, 118(10), 1659–1701. https://doi.org/10.1161/res.0000000000000097
Lopaschuk, G. D., Ussher, J. R., Folmes, C. D., Jaswal, J. S., & Stanley, W. C. (2010). Myocardial fatty acid metabolism in health and disease. Physiological Reviews, 90(1), 207–258. https://doi.org/10.1152/physrev.00015.2009
Jung, J., Wang, J., Groenendyk, J., Lee, D., Michalak, M., & Agellon, L. B. (2017). Fatty acid binding protein (Fabp) 5 interacts with the calnexin cytoplasmic domain at the endoplasmic reticulum. Biochemical and Biophysical Research Communications, 493(1), 202–206. https://doi.org/10.1016/j.bbrc.2017.09.046
Nguyen, H. C., Qadura, M., & Singh, K. K. (2020). Role of the fatty acid binding proteins in cardiovascular diseases: A systematic review. Journal of Clinical Medicine, 9(11), 3390. https://doi.org/10.3390/jcm9113390
Thumser, A. E., Moore, J. B., & Plant, N. J. (2014). Fatty acid binding proteins: tissue-specific functions in health and disease. Current Opinion in Clinical Nutrition and Metabolic Care, 17(2), 124–129. https://doi.org/10.1097/mco.0000000000000031
Storch, J., & Corsico, B. (2008). The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annual Review of Nutrition, 28(1), 73–95. https://doi.org/10.1146/annurev.nutr.27.061406.093710
Carbonetti, G., Wilpshaar, T., Kroonen, J., Studholme, K., Converso, C., d’Oelsnitz, S., & Kaczocha, M. (2019). FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Scientific Reports, 9(1), 18944. https://doi.org/10.1038/s41598-019-55418-x
Lv, Q., Wang, G., Zhang, Y., Han, X., Li, H., Le, W., Zhang, M., Ma, C., Wang, P., & Ding, Q. (2019). FABP5 regulates the proliferation of clear cell renal cell carcinoma cells via the PI3K/AKT signaling pathway. International Journal of Oncology, 54(4), 1221–1232. https://doi.org/10.3892/ijo.2019.4721
Pan, L., Xiao, H., Liao, R., Chen, Q., Peng, C., Zhang, Y., Mu, T., & Wu, Z. (2018). Fatty acid binding protein 5 promotes tumor angiogenesis and activates the IL6/STAT3/VEGFA pathway in hepatocellular carcinoma. Biomedicine and Pharmacotherapy, 106, 68–76. https://doi.org/10.1016/j.biopha.2018.06.040
Gally, F., Kosmider, B., Weaver, M. R., Pate, K. M., Hartshorn, K. L., & Oberley-Deegan, R. E. (2013). FABP5 deficiency enhances susceptibility to H1N1 influenza A virus-induced lung inflammation. American Journal of Physiology: Lung Cellular and Molecular Physiology, 305(1), L64-72. https://doi.org/10.1152/ajplung.00276.2012
Rao, D. M., Phan, D. T., Choo, M. J., Owen, A. L., Perraud, A. L., & Gally, F. (2019). Mice lacking fatty acid-binding protein 5 are resistant to listeria monocytogenes. Journal of Innate Immunity, 11(6), 469–480. https://doi.org/10.1159/000496405
Maeda, K., Uysal, K. T., Makowski, L., Görgün, C. Z., Atsumi, G., Parker, R. A., Brüning, J., Hertzel, A. V., Bernlohr, D. A., & Hotamisligil, G. S. (2003). Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes, 52(2), 300–307. https://doi.org/10.2337/diabetes.52.2.300
Li, Y., Li, Z., Zhang, C., Li, P., Wu, Y., Wang, C., Bond Lau, W., Ma, X. L., & Du, J. (2017). Cardiac fibroblast-specific activating transcription factor 3 protects against heart failure by suppressing MAP2K3-p38 signaling. Circulation, 135(21), 2041–2057. https://doi.org/10.1161/circulationaha.116.024599
Kubli, D. A., Zhang, X., Lee, Y., Hanna, R. A., Quinsay, M. N., Nguyen, C. K., Jimenez, R., Petrosyan, S., Murphy, A. N., & Gustafsson, A. B. (2013). Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. Journal of Biological Chemistry, 288(2), 915–926. https://doi.org/10.1074/jbc.M112.411363
Zhang, W., St Clair, D., Butterfield, A., & Vore, M. (2016). Loss of mrp1 potentiates doxorubicin-induced cytotoxicity in neonatal mouse cardiomyocytes and cardiac fibroblasts. Toxicological Sciences, 151(1), 44–56. https://doi.org/10.1093/toxsci/kfw021
Gu, X., Ma, Y., Liu, Y., & Wan, Q. (2021). Measurement of mitochondrial respiration in adherent cells by Seahorse XF96 Cell Mito Stress Test. STAR Protocols, 2(1), 100245. https://doi.org/10.1016/j.xpro.2020.100245
Li, B., Hao, J., Zeng, J., & Sauter, E. R. (2020). SnapShot: FABP functions. Cell, 182(4), 1066-1066.e1061. https://doi.org/10.1016/j.cell.2020.07.027
Gibb, A. A., Lazaropoulos, M. P., & Elrod, J. W. (2020). Myofibroblasts and fibrosis: mitochondrial and metabolic control of cellular differentiation. Circulation Research, 127(3), 427–447. https://doi.org/10.1161/circresaha.120.316958
Furuhashi, M., Ogura, M., Matsumoto, M., Yuda, S., Muranaka, A., Kawamukai, M., Omori, A., Tanaka, M., Moniwa, N., Ohnishi, H., Saitoh, S., Harada-Shiba, M., Shimamoto, K., & Miura, T. (2017). Serum FABP5 concentration is a potential biomarker for residual risk of atherosclerosis in relation to cholesterol efflux from macrophages. Scientific Reports, 7(1), 217. https://doi.org/10.1038/s41598-017-00177-w
Furuhashi, M., Sakuma, I., Morimoto, T., Higashiura, Y., Sakai, A., Matsumoto, M., Sakuma, M., Shimabukuro, M., Nomiyama, T., Arasaki, O., Node, K., & Ueda, S. (2020). Independent and distinct associations of FABP4 and FABP5 with metabolic parameters in type 2 diabetes mellitus. Frontiers in Endocrinology, 11, 696. https://doi.org/10.3389/fendo.2020.575557
Abplanalp, W. T., John, D., Cremer, S., Assmus, B., Dorsheimer, L., Hoffmann, J., Becker-Pergola, G., Rieger, M. A., Zeiher, A. M., Vasa-Nicotera, M., & Dimmeler, S. (2021). Single-cell RNA-sequencing reveals profound changes in circulating immune cells in patients with heart failure. Cardiovascular Research, 117(2), 484–494. https://doi.org/10.1093/cvr/cvaa101
van Bilsen, M., Smeets, P. J., Gilde, A. J., & van der Vusse, G. J. (2004). Metabolic remodelling of the failing heart: The cardiac burn-out syndrome? Cardiovascular Research, 61(2), 218–226. https://doi.org/10.1016/j.cardiores.2003.11.014
Rosca, M. G., Tandler, B., & Hoppel, C. L. (2013). Mitochondria in cardiac hypertrophy and heart failure. Journal of Molecular and Cellular Cardiology, 55, 31–41. https://doi.org/10.1016/j.yjmcc.2012.09.002
Lee, G. S., Pan, Y., Scanlon, M. J., Porter, C. J. H., & Nicolazzo, J. A. (2018). Fatty acid-binding protein 5 mediates the uptake of fatty acids, but not drugs, into human brain endothelial cells. Journal of Pharmaceutical Sciences, 107(4), 1185–1193. https://doi.org/10.1016/j.xphs.2017.11.024
Scharwey, M., Tatsuta, T., & Langer, T. (2013). Mitochondrial lipid transport at a glance. Journal of Cell Science, 126(Pt 23), 5317–5323. https://doi.org/10.1242/jcs.134130
Tuomainen, T., & Tavi, P. (2017). The role of cardiac energy metabolism in cardiac hypertrophy and failure. Experimental Cell Research, 360(1), 12–18. https://doi.org/10.1016/j.yexcr.2017.03.052
Hughes, C. S., ChinAleong, J. A., & Kocher, H. M. (2020). CRABP2 and FABP5 expression levels in diseased and normal pancreas. Annals of Diagnostic Pathology, 47, 151557. https://doi.org/10.1016/j.anndiagpath.2020.151557
Levi, L., Lobo, G., Doud, M. K., von Lintig, J., Seachrist, D., Tochtrop, G. P., & Noy, N. (2013). Genetic ablation of the fatty acid-binding protein FABP5 suppresses HER2-induced mammary tumorigenesis. Cancer Research, 73(15), 4770–4780. https://doi.org/10.1158/0008-5472.Can-13-0384
Koczor, C. A., Torres, R. A., Fields, E., Qin, Q., Park, J., Ludaway, T., Russ, R., & Lewis, W. (2013). Transgenic mouse model with deficient mitochondrial polymerase exhibits reduced state IV respiration and enhanced cardiac fibrosis. Laboratory Investigation, 93(2), 151–158. https://doi.org/10.1038/labinvest.2012.146
Jain, M., Rivera, S., Monclus, E. A., Synenki, L., Zirk, A., Eisenbart, J., Feghali-Bostwick, C., Mutlu, G. M., Budinger, G. R., & Chandel, N. S. (2013). Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. Journal of Biological Chemistry, 288(2), 770–777. https://doi.org/10.1074/jbc.M112.431973
Field, C. S., Baixauli, F., Kyle, R. L., Puleston, D. J., Cameron, A. M., Sanin, D. E., Hippen, K. L., Loschi, M., Thangavelu, G., Corrado, M., Edwards-Hicks, J., Grzes, K. M., Pearce, E. J., Blazar, B. R., & Pearce, E. L. (2020). Mitochondrial integrity regulated by lipid metabolism is a cell-intrinsic checkpoint for Treg suppressive function. Cell Metabolism, 31(2), 422-437. https://doi.org/10.1016/j.cmet.2019.11.021
Acknowledgements
We are grateful to Professor Moshi Song (Chinese Academy of Sciences, Beijing, China) for their helpful advice.
Funding
This study was supported by grants from the National Science Foundation of China (Grant Number, 81790622), and Beijing Collaborative Innovative Research Center for Cardiovascular Diseases.
Author information
Authors and Affiliations
Contributions
S.G., C.Z., Y.L., and J.D. conceived the project, designed the experiments, and wrote the paper. S.G., G.L., Y.S., Z.W., and S.H. performed the in vivo work. S.G., F.Q., and Y.J. performed the in vitro work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for Publication
All authors involved in this manuscript consent to its publication in Cardiovascular Toxicology in accordance with the journal's rules and regulations.
Ethics Approval
All animal experiments were approved by the Animal Care and Utilization Committee of Capital Medical University and performed in accordance with the guidelines of the Capital Medical University.
Additional information
Handling Editor: Y. James Kang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, S., Li, G., Shao, Y. et al. FABP5 Deficiency Impairs Mitochondrial Function and Aggravates Pathological Cardiac Remodeling and Dysfunction. Cardiovasc Toxicol 21, 619–629 (2021). https://doi.org/10.1007/s12012-021-09653-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09653-2