Abstract
Medicinal plants comprise a spectrum of constituents, encompassing both organic and inorganic elements. Elemental composition of 27 species of medicinal plants of Lamiaceae (including 17 endemic) family grown in Turkey was carried out by ICP-MS. The following elements were determined in analysed samples: Na, Mg, Al, K, Ca, Sc, Cr, Mn, Fe, Co, Zn, As, Rb, Sr, Cs, Ba, La, Ce, Sm, U, Se. Quantitative analysis of specific primary and secondary metabolites was carried out. Na and K are major constituents in plants. The concentrations of Na range from 332,495.590 g/kg (in sample 10SA) to 279,690.674 g/kg (in sample 4SA), while those of K vary from 67,492.456 g/kg (in sample 15SA) to 3347.612 g/kg (in sample 1A). Some metals such as Al, Cr, Mn, Fe, Co, Zn, As, Se, Rb, Sr, Cs, and Ba were also detected. Flavonoids, carbohydrates and tannins were present in all sample. Saponins were found in all samples except 1C and 2O. Coumarin were detected in samples 2N, 1 T, 1O, 1Z, 3SA, 1C, 4SA, 6SA, 8SA, 1 M, 11SA, 13SA, 2O, 14SA, 1H, and 16SI. Lipids were present in samples 6S, 9S, 1A, 10S, 1 M, 11SA, 12SA, 13SA, 14SA, and 16SI. Plants contain essential, rare earth, and trace elements at mg/kg concentrations, while major elements such as K and Na are present in high levels. Toxic element As (arsenic) was detected in all analyzed plants, but in most samples, its concentration was below the threshold set by World Health Organization.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicinal plants comprise a spectrum of constituents, encompassing both organic and inorganic elements. The presence of macronutrients and trace (micro-) elements in medicinal plants serves as a rich source, playing a pivotal role in preventing a myriad of diseases [1]. Herbal remedies derived from medicinal plants have been employed as therapeutic agents since ancient times. Various plant components, including leaves, flowers, stems, roots, seeds, and bark, are utilized either individually or in synergistic combinations for their medicinal properties. The efficacy of these plants lies in their bioactive phytochemical constituents, which elicit specific physiological responses in the human body. Phytochemicals can be broadly categorized into two groups based on their functions within the plant. Primary metabolites (PMs), such as sugars, amino acids, proteins, lipids, and chlorophyll, are essential for basic growth processes. In contrast, secondary metabolites (SMs), including alkaloids, essential oils, flavonoids, tannins, terpenoids, saponins, phenolic compounds, and cardiac glycosides, play a crucial role in the plant's defense mechanisms against herbivores and other inter-species threats. These SMs contribute to the intricate pharmacological profile of medicinal plants, making them valuable resources in traditional and modern medicine alike. SMs in plants serve not only as a diverse array of natural products but also play a crucial role in fortifying the plant's defense mechanisms against pathogenic assaults and environmental stressors. Possessing noteworthy biological activities, these plant SM are gaining prominence as key components in medicinal formulations and food additives, catering to therapeutic, aromatic, and culinary needs. Research into plant secondary metabolites has witnessed a steady rise over the past five decades [2,3,4,5].
In the realm of agricultural science, prevailing challenges affecting global crop production encompass issues related to nutrient management, the presence of heavy and toxic metals, and the quest for optimal plant productivity. Plants, enduring for numerous decades, exhibit a diverse composition of elements in varying proportions. The elements present in plants are broadly categorized as either macronutrients, including Ca, K, Mg, N, P, and S, or micronutrients such as B, Cu, Cl, Fe, Mn, Mo, Na, and Zn. These elements play a pivotal role in the developmental processes and growth of plants, underscoring their significance in the agricultural landscape [6]. Plants frequently assimilate nutrients in quantities surpassing their immediate requirements, necessitating the storage of these excess nutrients within plant tissues until they are needed. Despite the selective uptake of essential micro- and macronutrients and the implementation of sophisticated exclusion mechanisms, plants unavoidably absorb elements that possess toxicity [7]. Elements exist in various forms in nature, and their presence is indispensable for the body to execute diverse functions. Trace elements hold paramount importance in facilitating cellular functions at biological, chemical, and molecular levels. These elements play a pivotal role in orchestrating important biochemical reactions by serving as cofactors for numerous enzymes and acting as central components for stabilizing the structures of enzymes and proteins. Certain elements exert control over crucial biological processes by binding to molecules on the receptor sites of cell membranes or by altering the membrane structure to impede the entry of specific molecules into the cell. The functions of trace elements exhibit a dual nature; at normal levels, they are crucial for stabilizing cellular structures, yet in deficiency states, they may stimulate alternative pathways, leading to various diseases. Disruptions in the balance of trace elements can lead to the onset of pathological conditions and diseases [8, 9].
The Lamiaceae, commonly known as the mint family, exhibits a broad distribution across various natural ecosystems, encompassing 236 genera. Characterized by square stems in cross-section, opposite leaves, and zygomorphic flowers with five united petals and sepals, these aromatic plants are cultivated for their straightforward propagation through methods such as stem cutting [10]. The Lamiaceae (Labiatae) family boasts numerous medicinal plants of considerable value. Within this family, there are an estimated 6900 to 7200 species. Among the most prolific genera are Salvia (with approximately 900 species), Scutellaria (360 species), Stachys (300 species), Plectranthus (300 species), Hyptis (280 species), Teucrium (250 species), Vitex (250 species), Thymus (220 species), and Nepeta (200 species) [11]. Lamiaceae stands as the third-largest family in terms of taxon number and the fourth-largest based on species count in Turkey. With 48 genera and a total of 782 taxa (603 species, 179 subspecies, and varieties), 346 of these taxa (comprising 271 species, 75 subspecies, and varieties) are endemic, constituting approximately 44% of the family's diversity in the country (data updated as of February 1, 2017). Additionally, there are 23 hybrid species, 19 of which exhibit endemism (82%). These findings underscore Turkey's significance as one of the primary centers of diversity for Lamiaceae in the Old World. The flora of Turkey holds significant importance for the Lamiaceae family [10, 12]. Lamiaceae is celebrated for housing a variety of active secondary metabolites that hold substantial biological and economic significance. These compounds encompass volatile oils containing monoterpenes and sesquiterpenes, diterpenes, triterpenes, phenolic acids, flavonoids, and other substances, each contributing diverse properties [13].
The majority of species within this plant family are known for their aromatic qualities and contain essential oils, rendering them highly valuable in the fields of cosmetics, perfumery, agriculture, food, and medicine [14]. Throughout history, the species belonging to the Lamiaceae family have held a storied tradition of utilization for flavoring, food preservation, and medicinal applications, owing to their dual benefits of healing and preventative attributes. It is widely acknowledged that each species possesses a unique and intricate blend of bioactive compounds, where each component plays a role in its overall bioactivity. These plants are highly esteemed for their capability to produce an extensive array of secondary metabolites, showcasing potent antibacterial, antioxidant, anti-inflammatory, antimicrobial, antiviral, and anticancer activities [15].
The trace elements are presently recognized and classified by the World Health Organization (WHO) into three groups: essential elements, elements considered likely to be essential, and elements with potential toxicity. Man necessitates essential trace elements in quantities spanning from 50 µg to 18 mg per day, wherein they function as catalytic or structural components within larger molecules [16]. In our study, the major elements identified in Lamiaceae plants are sodium (Na) and potassium (K). Sodium and potassium play indispensable roles in human health, serving as crucial ions within the body and being intricately linked to numerous physiological and pathophysiological processes [17].
In this research, we undertook an evaluation of various elements in 27 (including 17 endemic) herb species belonging to the Lamiaceae family, gathered in Turkey, utilizing ICP-MS analysis. Moreover, a quantitative analysis of specific primary and secondary metabolites was carried out. A substantial number of the analyzed herbs are readily available and widely utilized voluntarily by the entire population of the country.
Material and Method
Plant Materials
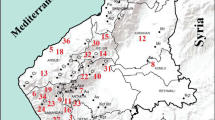
The names of the plants used, the localities where they were collected, their herbarium numbers and endemism status are given in Table 1. A map showcasing the plant collecting sites across Turkey is presented in Fig. 1.
Extraction
The extraction process utilized in the study involved a mobile maceration technique, wherein 10 g of the dried flowering above-ground parts of each plant were meticulously weighed and then transformed into powder form. Subsequently, this powdered plant material was subjected to a mobile maceration process with 150 ml methanol (after 8 h, it was filtered and 150 ml of methanol was added again and this process was continued for 3 days), which typically involves soaking the plant material in a solvent at room temperature for a specific duration. During the mobile maceration process, the powdered plant material was immersed in a suitable solvent. The choice of solvent can vary depending on the specific compounds being targeted for extraction and their solubility characteristics. Commonly used solvents for maceration with methanol. The duration of maceration typically ranged from 3 to 8 h at room temperature. This duration allows sufficient time for the solvent to penetrate the plant material and extract the desired compounds. After the designated maceration period, the filtrates obtained from each day's extraction were systematically combined. Subsequently, the combined filtrates underwent evaporation using a rotavapor. Rotavapor, short for rotary evaporator, is a common laboratory instrument used for gentle evaporation of solvents from a mixture under reduced pressure. This process helps concentrate the extracted compounds, leaving behind a more concentrated extract for further analysis. Overall, the mobile maceration process employed in the study involved soaking the powdered plant material in a solvent for a specific duration at room temperature to facilitate the extraction of bioactive compounds. This method allows for efficient extraction while preserving the integrity of the extracted compounds. At the conclusion of each day, the filtrates were systematically amalgamated and subjected to evaporation using a rotavapor.
Qualitative Analysis of Secondary and Primary Metabolites
Detection of Alkaloids
The methanolic extract of each species was weighed 0.5 g, 10 ml of 70% ethanol solution (containing 6% H2SO4) was added, boiled for 1 min and was cooled. Some of the extract was separated into 2 tubes and Mayer and Dragendorff reagents were added. The samples were checked for the appearance of precipitates and further experiments were continued for the samples in which precipitates were observed. After this control, the ethanol extract was alkalinized with a 25% Na2CO3 solution. Then, extracted with 15 ml of chloroform, the chloroformed layer was taken into a clean separating funnel and extracted with 15 ml of 10% acetic acid solution. The acetic acid phase was divided into three separate tubes and one tube was kept as a control, the second tube added Mayer's reagents and the third tube added Dragendorff's reagents. It was checked for the precipitate formation [18,19,20].
Detection of Flavonoids
The methanolic extract of each species was weighed 0.5 g and extracted with water. The test solution was extracted with ethyl acetate into a separatory funnel and the ethyl acetate phase was capsulated and dried in a water bath. The residue remaining in the capsule was dissolved with a mixture of HCl:MeOH:H2O (1:1:1) and transferred to a test tube. After adding Mg powder, the color was observed and the flavonoid was identified [18,19,20].
Detection of Cardioactive Heterosides
The methanolic extract of each species was weighed 0.5 g and prepared as a solution with 10 ml 70% ethanol. Add 1 ml of concentrated lead subacetate solution to the solution, mixed and filtered. The filtrate was extracted with 10 ml of chloroform, the chloroform layer was separated, taken into a capsule and evaporated. Add 3 ml of 3.5% glacial acetic acid solution of FeCl3 to the capsule and carefully transfer to 2 ml of concentrated sulfuric acid in a test tube to form a layer. The colors seen on the surface of the separation and in the acetic acid layers were observed [18,19,20].
Detection of Saponosides
The methanolic extract of each species was taken 0.5 g and placed in a test tube with 10 ml of hot water. After cooling, the tube was shaken strongly for 10 s. If saponoside was present, a 1–10 cm high foam layer was observed which remained stable for at least 10 min. Then 1–2 drops of 2N HCl were added and the persistence of the foam layer was observed. The methanolic extract of 0.5 g of each species was dissolved in chloroform and filtered. Add an equal volume of concentrated H2SO4 to the filtrate. The presence of fluorescence color in the chloroform layer and in the acidic layer was observed. The reaction is based on the coloration of the [18,19,20].
Detection of Anthocyanosides
The methanol extract of each species was extracted in 50% ethanol. The extract was filtered, the filtrate was separated into five portions and the following reactions were performed:
-
The addition of diluted H2SO4 resulted in the formation of red color.
-
First NaOH solution was added, then diluted HCl was added and the colors were observed.
-
The precipitate was observed with a 10% lead acetate solution.
-
Added a volume of amyl alcohol, shaken and observed the coloration of the layers [18,19,20].
Detection of Tannins
The methanol extract of each species was taken in a spatula to make an aqueous extract. The extract was divided into 3 parts and the following reactions were applied:
-
2 ml of 1% 1% saline gelatin solution (saturated with NaCl) was added and the precipitate was observed.
-
Tannins give a color reaction with iron salts. This reaction was determined with 5% FeCl3. Olive green and blue-black color formation was observed.
-
Brominated water was added and the precipitate were observed [18,19,20].
Detection of Anthracene Heterosides
The methanol extract of each species was taken at 0.5 g and boiled with diluted H2SO4. After filtering while hot, the filtrate was cooled. The extract was extracted with ether to eliminate the ether layer and shaken with 10% ammonia solution. The color in the underlying ammonia layer was observed [18,19,20].
Detection of Coumarin
The methanol extract of each species was taken 0.5 g, extracted in 10 ml ethanol and filtered. The filtrate was concentrated to dryness in a capsule and 1N NaOH solution was added. It was transferred into a tube and checked for fluorescence color at UV 366 nm [18,19,20].
Detection of Starch
The methanol extract of each species was taken 0.5 g and aqueous extract was prepared. Add a few drops of 0.1 N Iodine solution. The formation of purple color was checked [18,19,20].
Detection of Lipid
The extract of each species was made with petroleum ether and 5 ml was taken in a glass tube. It was concentrated in a water bath and applied as a stain on a filter paper. The filter paper was allowed to dry in an oven at 100 °C for 5 min. The oily stain was observed on the paper [18,19,20].
Detection of Carbohydrates
The methanol extract of each species was weighed 1 g and 6 ml of water was added to make an aqueous extract. It was filtered and the filtrate was divided into 3 tubes. The following experiments were applied to the solutions in the tubes respectively:
-
2 ml of Fehling A and 2 ml of Fehling B solutions were added to an empty tube and mixed. Then the test solution was added to it. The tube was heated and red colored precipitate.
-
1–2 drops of 15% solution of 1-naphthol in ethanol was added to 1 ml of the test solution. To this mixture, 1 ml of concentrated H2SO4. Purple ring formation was observed between the two layers.
-
Add a few crystals of resorcin to 1 ml of test solution. To this 1 ml of concentrated HCl was added and kept in a water bath. The presence of red color was observed [18,19,20].
Detection of Cyanogenetic Heterosites
0.5 g of the methanol extract of each species was taken into a flask and some water was added. The filter paper was wetted first with picric acid and then with sodium carbonate solution. The wetted filter paper was dropped into the flask. The flask was heated and red color formation was observed on the yellow colored filter paper [18,19,20].
ICP-MS Analysis of 21 Elements
The determination of elemental concentrations in the solution was carried out using an Inductively Coupled Plasma-Mass Spectrometer (ICP-MS), specifically the Agilent 7800 series provided by Agilent Technologies in Japan. For sample introduction, a glass MicroMist nebulizer from U-series in Australia and a double-pass quartz spray chamber from the USA were employed. The plasma system included a quartz torch (2.5 mm) and nickel components, including a sample cone and skimmer cone for the x-lens. Before sample analysis, all quartz and nickel components underwent a meticulous cleaning process. Quartz and glass elements were soaked in a 5–10% HNO3 solution overnight, thoroughly rinsed with distilled water, dried in an oven, and then installed on the device. Nickel components, such as sample and skimmer cone pieces, underwent ultrasonic baths in pure water, 5% HNO3 solution, and distilled water successively for five minutes each. Following this, they were cleaned with a cotton ball, rinsed thoroughly with distilled water, and dried in the oven before installation on the device. Before analysis, the device underwent a 45-min helium gas purge. Activation was followed by adjusting parameters, including Plasma gas (15 L/min), auxiliary gas (1 L/min), carrier gas (1 L/min), makeup/dilution gas (1 L/min), and carrier gas pressure (1.45 kPa). The plasma gas, auxiliary gas, carrier gas, and makeup/dilution gas were Argon (Ar).Torch axis, resolution axis, EM, standard lenses tune, plasma correction, full spectrum, and performance report tests were carried out sequentially. Calibration of the device utilized a tuning solution (1 µg/L Ce, Co, Li, Mg, Tl, Y). The values obtained during tuning were scrutinized to identify any deviations in the device. Standard solutions, prepared from stock solutions, were then analyzed, and calibration curves were verified within the standard reference range (0–10, 25, 50, 100, 250, 500 g/kg, mg/kg, and µg/kg for Na, Mg, Al, K, Ca, Sc, Cr, Mn, Fe, Co, Zn, As, Se, Rb, Sr, Cs, Ba, La, Ce, Sm, and U elements.
Results
Qualitative Analysis of Secondary Metabolites
The qualitative analysis of primary and secondary metabolites for the samples is detailed in Table 2. Flavonoids were identified in all samples, while tannins were present in every sample. Saponins were found in all samples except 1C and 2O. Coumarin was detected in various samples, including 2N, 1 T, 1O, 1Z, 3SA, 1C, 4SA, 6SA, 8SA, 1 M, 11SA, 13SA, 2O, 14SA, 1H, and 16SI. Cardioactive heterosides were absent in all samples. Starch was identified solely in samples 4SA and 10SA. Lipids were present in samples 6S, 9S, 1A, 10S, 1 M, 11SA, 12SA, 13SA, 14SA, and 16SI, while carbohydrates were observed in all samples. Notably, no alkaloids, anthocyanosides, anthracene heterosides, or cyanogenetic heterosides were detected among the samples. The qualitative analysis data of primary and secondary metabolites for the methanolic extracts were provided in Table 2.
Elemental Analysis
The analysis of the elemental composition of methanol extracts revealed notable variations in element concentrations, as outlined in Table 3. Using ICP-MS, the methanol extracts were investigated for the presence of 21 elements, including Na, Mg, Al, K, Ca, Sc, Cr, Mn, Fe, Co, Zn, As, Rb, Sr, Cs, Ba, La, Ce, Sm, U, and Se. The results indicated elevated levels of Na [ranging from 332,495.590 g/kg (in sample 10SA) to 279,690.674 g/kg (in sample 4SA) g/kg] and K [ranging from 67,492.456 g/kg (in sample 15SA) to 3347.612 g/kg (in sample 1A)]. Some heavy metals such as Al, Cr, Mn, Fe, Co, Zn, As, Se, Rb, Sr, Cs, Ba, La, Ce, Sm (except samples 1SI, 2SA-12SA), and U (including samples 15SA and 16 SI) were also detected.
Discussion
Qualitative Analysis of Secondary and Primary Metabolites
A comprehensive review of literature from 2002 to 2018 revealed various classes of secondary metabolites in this family, including flavonoids, fatty derivatives, and sterols [13].
In a review article, a total of 217 articles were selected from the initial search focusing on Lamiaceae, specifically those recognizing Mexican genera and species. The bioactive constituents identified in these genera predominantly include terpenes (both volatile and non-volatile) and phenolic compounds, particularly flavonoids in the form of glycosides and aglycones [22].
A review provided an overview of Nepeta species, focusing on their phytochemical characteristics. Terpenoids and phenolic compounds were predominantly identified through the application of chromatographic and spectroscopic techniques [23]. In another review, major constituents as nepetalactones, iridoids and their glucosides, diterpenes, triterpenes, and flavonoids, as well as essential oil, have been identified within Nepeta species [24]. The phytochemical composition and trace elements of Nepeta suavis were examined in an analysis. The findings unveiled the existence of bioactive components, including flavonoids, alkaloids, phenols, saponins, and tannins. Furthermore, the herb proved to be a rich reservoir of essential minerals such as Na, K, Ca, Mg, Zn, Fe, and P [25]. The volatile oils stand out as the primary constituents of the Thymus genus. In addition to these, the genus is rich in flavonoids, phenylpropanoids, tannins, organic acids, terpenoids, and phytosterols [26]. The Origanum genus exhibits chemical variations. Oregano, recognized for its distinctive flavor, is primarily associated with several plant species known for producing essential oils rich in carvacrol. Additionally, the genus consists of a diverse range of compounds, including terpenes, phenols such as phenolic acids, and flavonoids [27]. Numerous chemical constituents have been elucidated within the Sideritis genus, encompassing terpenes, flavonoids, essential oils, iridoids, coumarins, lignans, and sterols. Diterpenes, flavonoids, and essential oils are consistently present in nearly every species, serving as the primary components responsible for the pharmacological activity [28].
In a study, the aerial parts of Sideritis and Origanum species were analyzed for mineral contents, flavonoids, total phenols, and anthocyanins. K was found to be high in both plant species. In Sideritis, K contents ranged from 10,184.91 mg/kg (Sideritis libanotica subsp. linearis) to 17,182.86 mg/kg (S. hispida), while in Origanum, they ranged from 10,265.40 mg/kg (Origanum majorana) to 21,293.79 mg/kg (O. vulgare). Crude protein contents in Sideritis varied between 1.55% (S. libanotica subsp. linearis) and 7.83% (S. perfoliata), whereas protein contents in Origanum species ranged from 1.99% (O. leptocladum) to 5.51% (O. vulgare). Flavonoid contents in Sideritis plants ranged from 246.34 (S. libatotica subsp. linearis) to 2013.33 (S. hololeuca), and in Origanum plants, they varied from 345.38 (O. onites) to 1730.47 (O. majorana). For Origanum, K contents ranged between 10,265.40 mg/kg (O. majorana) and 21,293.79 mg/kg (O. vulgare) [29].
The terpenoids and flavonoids constitute the primary secondary metabolite constituents of Salvia, with over 80% being terpenoids, particularly abietane and clerodane diterpenoids. Notably, sesquiterpenoids and triterpenoids are relatively scarce in Salvia species. Numerous studies highlight the presence of flavonoids, triterpenoids, and monoterpenes, particularly in the flowers and leaves, while diterpenoids are predominantly found in the roots. However, literature surveys indicate that certain American Salvia species also contain diterpenoids in aerial parts, and in some Salvia species, triterpenoids and flavones are present in the roots [30]
In a research, Salvia officinalis, S. sclarea, S. pratensis, and S. nemorosa originating from Hungary and Transylvania were investigated for their tannin and flavonoid content. Significant differences (p < 0.05) were observed in tannin content between Hungarian and Transylvanian S. officinalis and flavonoid content between Hungarian and Transylvanian S. sclarea. Chromium content was notably high in all examined species. The element concentrations also differed significantly in aqueous extracts, with distinct dissolution rates. Notably, Hungarian S. officinalis exhibited elevated concentrations of Al, Fe, Mn, and Ti, possibly linked to soil pollution. Zinc accumulation was highest in Transylvanian S. officinalis and S. pratensis, while Hungarian S. nemorosa demonstrated elevated Li content. Chromium content was notably high in Hungarian S. officinalis and S. sclarea. Dissolution rates of elements varied widely among sage teas, showcasing significant differences in element concentrations (Al, B, Ba, Cu, Fe, K, Mg, Mn, Na, P, S, Zn) and dissolution characteristics based on the sample and the element studied [31].
The genus Ziziphora has been extensively studied, and previous reports in the literature highlight its species as rich sources of valuable bioactive compounds, including sterols, fatty acids, caffeoyl derivatives, and flavonoids. Notably, among the different groups of natural compounds, flavonoids, flavones, and their derivatives exhibit the highest frequency in the separated and characterized bioactive compounds, contributing significantly to each profile [32].
In literature, studies have indicated that Cyclotrichium essential oils are abundant in phenolic and alcoholic compounds, along with monoterpenes. Phenolic compounds are widely believed to be abundant in most Cyclotrichium species. C. origanifolium is rich in flavonoid [33]. According to our knowledge, this is the first elemental analysis conducted on the Cyclotrichium genus.
A comprehensive review aimed to provide an in-depth summary of the genus Ajuga L. Currently, more than 280 chemical constituents have been isolated and characterized from Ajuga species, with neo-clerodane diterpenes and diterpenoids, phytoecdysteroids, flavonoids, and iridoids identified as the major bioactive compounds [34]. This study represents the inaugural investigation into the elemental analysis of Ajuga chamaepitys.
In a comprehensive literature review on the genus Marrubium, chemical characteristics were investigated. The biological effects of the plants are often attributed to the presence of diterpenes, sterols, phenylpropanoids, and flavonoids [35].
Hyssop (Hyssopus sp.) genus has been known with volatile oils. The genus also containe flavonoid in leaves, flowers, stalks, and roots during full flowering. Furthermore, hyssop seeds have fatty acid content in oil [36].
Elemental Analysis
In a study conducted similarly to our research, the elemental composition of 45 medicinal plant species belonging to the Lamiaceae family in the Republic of Moldova was investigated. Various elements, including essential, rare earth, and trace elements (measured in μg/L), as well as major elements such as Ca, Cl, K, Mg, and Na (measured in mg/L), were determined in the analyzed samples. Despite the presence of the toxic element As in all plants, its concentration in the majority of samples was below the limit set [37].
The study conducted in Pakistan, aimed to investigate the phytochemical, and elemental aspects of five crucial medicinal plants from the Lamiaceae family, namely Mentha longifolia, M. piperita, M. spicata, Ocimum basilicum, and Rosmarinus officinalis, collected in Peshawar district. Quantitative analysis of macro- and microminerals identified 13 elements (C, N, O, Mg, K, P, S, Ca, Al, Si, Fe, Cl, and Na) present in varying amounts among species. Methanol extracts from leaves were analyzed for phytochemical constituents such as saponins, flavonoids, tannins, terpenoids, phlobatannins, steroids, and anthraquinones [38].
Another research aimed to analyze the elemental content of six medicinal plant species from the Lamiaceae family, predominantly found in the western part of Romania. The findings indicate that these medicinal plants are rich sources of nutrients, with high potassium (K) content, followed by calcium (Ca), magnesium (Mg), iron (Fe), and zinc (Zn) [39].
A study explored the mineral elements of extract from N. italica subsp. cadmea. Mineral elements (P, Mg, K, Fe, Cu) were found in the extract [40]. A paper focused on the analysis of trace elements in the Indian medicinal plant Nepeta hindostana. The plant was identified as a rich source of essential trace elements as Na, K, Mg, Zn, Fe, and Mn [41].
A study investigated the concentrations of eleven mineral and trace elements (Mg, Ca, K, Fe, Mn, Al, Zn, Cu, Cd, Ni, and Pb) in various Thymus species in Turkey, including the Thymus cilicicus. The elemental concentrations (mg/g) in T. cilicicus were found to be 0.40 ± 0.02 (Pb), 0.20 ± 0.10 (Ni), 0.04 ± 0.01 (Cd), 10.70 ± 2.30 (Cu), 40.80 ± 4.70 (Zn), 191.0 ± 8.0 (Al), 19.0 ± 2.0 (Mn), 189.0 ± 2.0 (Fe), 12,250.0 ± 672.0 (K), 14,417.0 ± 1909.0 (Ca), and 4127.0 ± 1200.0 (Mg). T. cilicicus stands out as particularly rich in essential elements, with the highest concentrations observed for Ca, K, and Mg [42].
In a study, elemental concentrations in Ziziphora medicinal plants were analyzed. Samples were collected from the Eynali mountain region in the north of Tabriz, Iran. The study revealed varying concentrations of Al, Ca, K, Mg, Mn, Na, V, Cl, and Ti elements in powdered Ziziphora plants. Notably, Ziziphora plants exhibited richness in essential elements such as Mg, K, and Ca, all of importance for human health. Importantly, the concentration of the potentially toxic element, Al, was determined to be below the levels permitted by the World Health Organization (WHO) [43].
In research, to rationalize its medicinal applications and establish a biogeochemical link, the mineral elements (Na, K, Ca, Mg, Zn, Mn, Cu, Fe, Cr) present in the leaves and roots of Ajuga bracteosa were investigated. The herb exhibited comparatively higher amounts of chromium (25 mg per 100 g in leaves and 20 mg in roots), which may be associated with its traditional use as a remedy for diabetes. Additionally, the herb contains significantly higher levels of potassium (139 mg per 100 g in leaves and 159 mg in roots) compared to sodium (21 mg per 100 g in leaves and 29 mg in roots), suggesting a potential correlation with its use in managing hypertension [44].
According to our knowledge, this is the first elemental analysis conducted on the Marrubium globosum. In another study, instrumental neutron activation analysis identified twenty-two chemical elements in Marrubium vulgare. The study revealed that K was the dominant chemical element in the plant, constituting 4.40% of the mass. Ca and Fe mass fractions were also relatively high. Importantly, potential toxic elements in this Lamiaceae plant were found to be within the safety limits recommended by WHO/FAO [45].
A study considered four harvest heights (15, 25, 35, and 45 cm from the tip of the Hyssopus officinalis) along with the residual stalks. Dependent variables included the accumulated content of elements (N, K, P, Ca, Mg, Cu, Zn, and Pb) at different heights. Results revealed that moving from the upper shoots towards the ground led to an increase in Mg, Ca, Cu, Zn, and Pb content by 22.67%, 43.74%, 12.87%, 39.02%, and 85.04%, respectively. Conversely, a downward trend was observed for N (50.16%) and K (6.41%) content, while an upward trend was noted for P (29.06%) content. In residual stalks, moving from upper shoots to the ground resulted in decreased Mg, Ca, Cu, Zn, and Pb contents by 1.01%, 21.03%, 9.11%, 17.02%, and 51.06%, respectively. However, N and P contents increased by 60.59% and 3.15%, respectively, with a 34.74% increase in P content [46].
The comprehensive analysis of primary and secondary metabolites, alongside elemental composition evaluation of methanol extracts, offers profound insights into the characteristics and potential functionalities of the samples. The ubiquitous presence of flavonoids and tannins across all samples hints at the antioxidant capabilities and potentially broader health-promoting effects of the plants under study. Flavonoids, renowned for their antioxidant and anti-inflammatory properties, stand as focal points in numerous health studies. Conversely, tannins, recognized for antimicrobial and antiviral attributes, contribute to the overall pharmacological profile of these plants. The absence of cardioactive heterosides suggests a lack of pronounced effects on cardiac function, which could be advantageous or constraining depending on the intended application of these botanicals. The detection of coumarin in varied samples sparks interest due to its diverse pharmacological spectrum, encompassing anticoagulant, anti-inflammatory, and antimicrobial facets. This discovery implies that the plants may harbor therapeutic potential extending beyond mere antioxidant capacities. The presence of lipids in several samples and the occurrence of starch in select ones accentuate the nutritional diversity inherent in these botanicals. Lipids, vital constituents of cellular membranes, and starch, a pivotal energy source, contribute to the overall nutritional value of these plants. Regarding elemental composition, the elevated levels of sodium and potassium throughout the samples hint at their potential mineral richness. However, the identification of heavy metals like aluminum, chromium, and arsenic raises concerns regarding potential toxicity, particularly in samples exhibiting higher concentrations of these elements. The provided text offers a comprehensive overview of studies focusing on the Lamiaceae family, exploring the elemental composition and phytochemical characteristics of various plant species within this family. These studies shed light on the diverse array of bioactive compounds present in Lamiaceae plants, ranging from essential minerals to secondary metabolites like flavonoids, terpenoids, and phenolic compounds. One notable aspect highlighted in the text is the prevalence of essential elements such as K, Ca, Mg, and Fe across different Lamiaceae species. These elements play crucial roles in various physiological processes, including enzymatic reactions, cellular signalling, and structural support. The abundance of these minerals underscores the potential nutritional significance of Lamiaceae plants in human diets. Additionally, the presence of bioactive compounds like flavonoids, terpenoids, and phenolic compounds underscores the therapeutic potential of Lamiaceae plants. These compounds have been associated with various pharmacological activities, including antioxidant, anti-inflammatory, and antimicrobial properties. The diverse chemical profile of Lamiaceae plants suggests their potential utility in traditional medicine and as sources of novel therapeutic agents. Furthermore, the studies discussed in the text also address the elemental variability among different Lamiaceae species, as well as the influence of environmental factors on elemental composition. Understanding these variations is crucial for assessing the nutritional quality and potential health benefits of Lamiaceae plants, as well as for elucidating their ecological roles and adaptation strategies. Overall, the findings presented in the text underscore the importance of interdisciplinary approaches in studying plant chemistry and ecology. Integrating elemental analysis with phytochemical profiling provides valuable insights into the nutritional, medicinal, and ecological significance of Lamiaceae plants, ultimately contributing to our understanding of their roles in human health and natural ecosystems.
In summary, these findings underscore the intricate nature of plant metabolites and emphasize the necessity for exhaustive analysis in deciphering their potential health implications. Further investigations, encompassing pharmacological and toxicological inquiries, are imperative for a comprehensive understanding of the therapeutic prospects and safety profiles associated with these botanicals.
Conclusion
In conclusion, the comprehensive analysis of 27 medicinal plant species from the Lamiaceae family in Turkey revealed a diverse spectrum of constituents, encompassing both organic and inorganic elements. The elemental composition, including 18 endemic species, was determined using ICP/MS, highlighting the presence of essential, rare earth, and trace elements at mg/kg concentrations. Major elements such as Na and K were found in elevated levels, demonstrating the richness of these plants in important nutrients. Despite the widespread detection of the toxic element As (arsenic), the concentrations in most samples remained below the World Health Organization's established threshold, assuring the safety of these medicinal plants. The inclusion of specific primary and secondary metabolites, such as flavonoids, tannins, saponins, coumarin, starch, lipids, and carbohydrates, further underscored the diverse chemical composition of these plants. Overall, this study provides valuable insights into the elemental and chemical profiles of medicinal plants from the Lamiaceae family, contributing to our understanding of their potential therapeutic properties and nutritional benefits. The presence of essential elements in trace amounts, coupled with the relatively low levels of toxic elements, reinforces the potential of these plants as valuable resources for traditional and modern medicine.
Data Availability
Data will be made available on request.
References
Jyothsna S, Manjula G, Suthari S, Rao AN (2020) Qualitative elemental analysis of selected potential anti-asthmatic medicinal plant taxa using EDXRF technique. Heliyon 6(2):e03260
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Saxena HO, Soni A, Mohammad N, Choubey SK (2014) Phytochemical screening and elemental analysis in different plant parts of Uraria picta Desv.: a Dashmul species. J Chem Pharm Res 6:756–760
Tiwari R, Rana CS (2015) Plant secondary metabolites: a review. Int J Eng Res Gen Sci 3:661–670
Yang L, Wen K-S, Ruan X et al (2018) Response of plant secondary metabolites to environmental factors. Molecules 23:762
Singh VK, Sharma N, Verma ON et al (2021) application of LIBS to elemental analysis and mapping of plant samples. Spectrosc 42:99–113
Conn S, Gilliham M (2010) Comparative physiology of elemental distributions in plants. Ann Bot 105:1081–1102
Prashanth L, Kattapagari KK, Chitturi RT et al (2015) A review on role of essential trace elements in health and disease. J Dr YSR Univ Health Sci 4:75–85
Skalnaya MG, Skalny AV (2018) Essential trace elements in human health: a physician’s view. Tomsk Publ House Tomsk State Univ 224:1–222
Ebadollahi A, Ziaee M, Palla F (2020) Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 25:1556
Karpiński TM (2020) Essential oils of Lamiaceae family plants as antifungals. Biomolecules 10:103
Selvi S, Polat R, Cakilcioglu U et al (2022) An ethnobotanical review on medicinal plants of the Lamiaceae family in Turkey. Turk J Bot 46:283–332
Hamed AN, Attia E, Desoukey SY (2021) A review on various classes of secondary metabolites and biological activities of Lamiaceae (Labiatae) (2002–2018). J Adv Biomed Pharm Sci 4:16–31
Valerio F, Mezzapesa GN, Ghannouchi A et al (2021) Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy 11:1431
Carović-StanKo K, PeteK M, Grdiša M et al (2016) Medicinal plants of the family Lamiaceae as functional foods-a review. Czech J Food Sci 34:377–390
Aliasgharpour M, Rahnamaye Farzami M (2013) Trace elements in human nutrition: a review. Int J Med Investig 2(3):115–128
Pohl HR, Wheeler JS, Murray HE (2013) Sodium and potassium in health and disease. In: Sigel A, Sigel H, Sigel RKO (eds) Interrelations between essential metal ions and human diseases. Springer, Dordrecht, pp 29–47
Karakaya S (2016) Investigations on Ferulago trachycarpa Boiss., F. blancheana Post., F.pachyloba (Fenzl) Boiss. and F. bracteata Boiss. & Haussk. (Apiaceae). Doctoral Thesis, Ankara University, Health Sciences Institute
Tanker M, Tanker N (1991) Farmakognozi, Ankara Üniversitesi Basımevi. A.Ü. Ecz. Fak. Yayınları, Ankara
Yuca H, Civas A, Tekman E et al (2023) Qualitative and quantitative phytochemicals of essential oils and extracts of Thymbra spicata subsp. spicata L. as a spice for diabetes mellitus. Flavour Fragr J 38:378–391. https://doi.org/10.1002/ffj.3753
Brown RJ, Milton MJ (2005) Analytical techniques for trace element analysis: an overview. TrAC, Trends Anal Chem 24(3):266–274. https://doi.org/10.1016/j.trac.2004.11.0
Hernandez-Leon A, Moreno-Pérez GF, Martínez-Gordillo M et al (2021) Lamiaceae in Mexican species, a great but scarcely explored source of secondary metabolites with potential pharmacological effects in pain relief. Molecules 26:7632
Suntar I, Nabavi SM, Barreca D et al (2018) Pharmacological and chemical features of Nepeta L. genus: its importance as a therapeutic agent. Phytother Res 32:185–198. https://doi.org/10.1002/ptr.5946
Formisano C, Rigano D, Senatore F et al (2008) Volatile constituents of aerial parts of three endemic Centaurea species from Turkey: Centaurea amanicola Hub.-Mor., Centaurea consanguinea DC. and Centaurea ptosimopappa Hayek and their antibacterial activities. Nat Prod Res 22:833–839. https://doi.org/10.1080/14786410701218259
Hussain J, Khan FU, Ullah R et al (2009) Evaluation of the chemical composition and trace elements analysis of Nepeta suavis and Phlomis bracteosa belonging to family Labitae. Am Eurasian J Agric Env Sci 6:651–656
Li X, He T, Wang X et al (2019) Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem Biodivers 16:e1900254. https://doi.org/10.1002/cbdv.201900254
Marrelli M, Statti GA, Conforti F (2018) Origanum spp.: an update of their chemical and biological profiles. Phytochem Rev 17:873–888. https://doi.org/10.1007/s11101-018-9566-0
González-Burgos E, Carretero ME, Gómez-Serranillos MP (2011) Sideritis spp.: Uses, chemical composition and pharmacological activities—a review. J Ethnopharmacol 135:209–225. https://doi.org/10.1016/j.jep.2011.03.014
Dursun N, Dogu S, Gezgin S et al (2016) Mineral contents and chemical properties of some Sideritis and Origanum species. J Agroaliment Process Technol 22:220–225
Wu Y-B, Ni Z-Y, Shi Q-W et al (2012) Constituents from Salvia species and their biological activities. Chem Rev 112:5967–6026. https://doi.org/10.1021/cr200058f
Szentmihályi K, Then M, Csedó (2004) Comparative study on tannins, flavonoids, terpenes and mineral elements of some Salvia species. Acta Hortic 463–470. https://doi.org/10.17660/ActaHortic.2004.629.60
Mohammadhosseini M (2017) The ethnobotanical, phytochemical and pharmacological properties and medicinal applications of essential oils and extracts of different Ziziphora species. Ind Crops Prod 105:164–192
Yazdanshenas H, Tafrihi M (2022) The biological and therapeutic potentials of Cyclotrichium genus: a systematic review. Int J Environ Health Res 32:2589–2599. https://doi.org/10.1080/09603123.2021.1977784
Luan F, Han K, Li M et al (2019) Ethnomedicinal uses, phytochemistry, pharmacology, and toxicology of species from the genus Ajuga L.: a systematic review. Am J Chin Med 47:959–1003. https://doi.org/10.1142/S0192415X19500502
Meyre-Silva C, Cechinel-Filho V (2010) A review of the chemical and pharmacological aspects of the genus Marrubium. Curr Pharm Des 16:3503–3518. https://doi.org/10.2174/138161210793563392
Jankovskỳ M, Landa T (2002) Genus Hyssopus L.-recent knowledge. Hort SciPrague 29:119–123
Zinicovscaia I, Gundorina S, Vergel K et al (2020) Elemental analysis of Lamiaceae medicinal and aromatic plants growing in the Republic of Moldova using neutron activation analysis. Phytochem Lett 35:119–127
Shah SM, Amin M, Gul B, Begum M (2020) Ethnoecological, elemental, and phytochemical evaluation of five plant species of Lamiaceae in Peshawar, Pakistan. Scientifica 2020:1–8
Tomescu A, Rus C, Pop G et al (2015) Researches regarding proximate and selected elements composition of some medicinal plants belonging to the Lamiaceae family. Lucr Ştiinţ 58:175–180
Kaska A, Cicek M, Mammadov R (2019) Biological activities, phenolic constituents and mineral element analysis of two endemic medicinal plants from Turkey: Nepeta italica subsp. cadmea and Teucrium sandrasicum. South Afr J Bot 124:63–70
Koche D (2010) Trace element analysis and vitamins from an Indian medicinal plant Nepeta hindostana (ROTH) Haines. Int J Pharm Pharm Sci 3:53–54
Kucukbay F, Kuyumcu E (2010) Determination of trace element contents of Thymus species from Turkey. Turk J Chem 34:911–920
Jooybari BS, Soltanpour H, Uchbelagh DR (2022) Multi element analysis of Ziziphora medicinal plant by instrumental neutron activation analysis. Presented at the 1st İnternational and 28th National Conference on Nuclear Science and Technology, Iran
Ahmed D, Chaudhary MA (2009) Medicinal and nutritional aspects of various trace metals determined in Ajuga bracteosa. J Appl Sci Res 5:864–869
Nedjimi B, Beladel B (2016) Instrumental neutron activation analysis of Marrubium vulgare L., a valuable medicinal herb. Radiochim Acta 104:285–289. https://doi.org/10.1515/ract-2015-2406
Saebi A, Minaei S, Mahdavian AR, Ebadi M-T (2021) Precision Harvesting of medicinal plants: elements and ash content of Hyssop (Hyssopus officinalis L.) as affected by harvest height. Biol Trace Elem Res 199:753–762. https://doi.org/10.1007/s12011-020-02171-2
Acknowledgements
Enes TEKMAN extends appreciation for the scholarship granted by the Turkish Scientific and Technical Research Council (TÜBİTAK) and acknowledges the assistance received during their postgraduate program.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Conceptualization: E.T., T.A., H.Y., A.A., Ö.Ç., S.K. Data curation: E.T., T.A., H.Y., A.A., Ö.Ç., S.K. Formal analysis and investigation: E.T., T.A., H.Y., A.A., S.K. Software: H.Y., S.K. Supervision: S.K. Resourches: Ö.Ç. Writing original draft: E.T., H.Y., S.K. Review and editing: H.Y., S.K. The manuscript has been checked and approved by all authors.
Corresponding author
Ethics declarations
Ethics Approval
There is no ethics approval.
Competing Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tekman, E., Asgarlı, T., Yuca, H. et al. Exploring Quantitative Biological Major, Trace, and Ultratrace Elements Composition and Qualitative Primary-Secondary Metabolites in Lamiaceae Medicinal Plants from Turkey. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04219-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04219-z