Abstract
The present study investigated the cytotoxic effects of ZnO, CuO, and mixed combinations of them on SH-SY5Y cells. For this purpose, the cells were exposed to various concentrations of these NPs alone for 24–96 h and as a mixture for 24 h. Variations in cell viability were noted. MTT results showed that ZnO and/or CuO NPs decreased cell survival by about 59% at 200 (ZnO, at 24 h) and 800 µg/ml (ZnO and/or CuO, at 72 and 96 h). When the NR assay was used, slight decreases were noted with ZnO NPs at 72 and 96 h. With CuO NPs alone and NPs in a mixture, only the highest concentrations caused 40 and 70% decreases in cell survival, respectively. Especially with NR assays, DTPA, NAC, or taurine provided marked protection. ROS levels were increased with the highest concentration of CuO NPs and with all concentrations of the mixture. The highest concentration of ZnO NPs and the lowest concentration of CuO NPs caused slight decreases in mitochondrial membrane potential levels. Additionally, increases were noted in caspase 3/7 levels with ZnO and CuO NPs alone or with a mixture of them. Intracellular calcium levels were decreased in this system. These findings demonstrated that ZnO and CuO NPs, either separately or in combination, had a modest cytotoxic effect on SH-SY5Y cells. Protection obtained with DTPA, NAC, or taurine against the cytotoxicity of these NPs and the ROS-inducing effect of CuO NPs and the NPs’ mixture suggests that oxidative stress might be involved in the cytotoxicity mechanisms of these NPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to human-made nanoparticles (NPs) including metal NPs is increasing rapidly in today’s world [1,2,3,4]. Considering the lack of exact information on their toxic effects, more studies are needed to show their possible unwanted effects in different model systems. On the other hand, organisms might be exposed to NPs as a mixture. As mixture studies are gaining importance in the toxicology area for years [5], investigations on the toxicity of a mixture of man-made NPs are also important.
The effects of man-made NPs on brain health is also a crucial research field, and available data suggest that the blood–brain barrier (BBB) cannot provide effective protection against these types of NPs [6]. Regarding the very special role, complex functions, and importance of the brain in the human organism on the one hand, and numerous unanswered questions on the toxicity of man-made NPs in different model systems on the other, there are raising concerns about potentially toxic effects of man-made NPs on the brain [6, 7].
ZnO and CuO NPs with their special properties are examples of the widely used metal NPs [8]. Their antiviral, antifungal, and antibacterial properties make them preferred among other NPs [8,9,10]. For instance, ZnO NPs are used in drug delivery, the pharmaceutical industry, and biomedical engineering, as well as in sunscreens, food additives, dental fillings, and cosmetics. And, CuO NPs are wielding in numerous biomedical applications, magnetic storage media, and the production of sensors, semiconductors, biosensors, and near-infrared filters [9,10,11,12,13,14,15]. As a result of their increasing usage, interaction possibilities between organisms and these two NPs are also rising [10, 16]. Additionally, there is not enough study regarding health risks due to exposure to a mixture of NPs. In a study performed by Parsai and Kumar [17], they indicate there is a lack of data on the interaction of toxicity of ZnO and CuO NPs. And in order to estimate the reference dose, and decide synergism and antagonism effects of these NPs/ion combinations, further information on the toxicity of these NPs is needed [17].
There are numerous in vitro studies on the cytotoxic effects of manufactured NPs in different central nervous system cells [18,19,20]. However, investigations on the cytotoxic effects of ZnO and CuO NPs are limited [4, 21,22,23,24,25,26].
Regarding the above-mentioned information, this study aims to determine the possible time- and dose-dependent cytotoxic effects of ZnO and CuO NPs alone or as a mixture on human neuroblastoma (SH-SY5Y) cells. The protective effects of diethylenetriaminepentaacetic acid (DTPA), N-acetylcysteine (NAC), and taurine against NPs-induced toxicity were also examined in the same model system. To the best of our knowledge, this is the first study to examine the toxic effects of mixtures of ZnO (< 100 nm) and CuO NPs (< 50 nm) on SH-SY5Y cells.
Materials and Methods
Chemicals
ZnO NPs (< 100 nm) and CuO NPs (< 50 nm) were obtained from Sigma-Aldrich (Missouri, USA). The cell culture solutions were from Biological Industries (Kibbutz Beit-Haemek, Israel) and Biowest (Nuaillé, France). Plastic culture materials were purchased from Corning (New York, USA) and Greiner (Kremsmünster, Austria). The other chemicals were obtained either from Sigma-Aldrich (Missouri, USA) or Merck (Darmstadt, Germany). 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide derivative (JC-10) and carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP) were from Enzo Life Sciences (Farmingdale, USA).
Preparation and Characterization of Nanoparticle Solutions
Stock solutions of ZnO (< 100 nm, Sigma) and CuO (< 50 nm, Sigma) NPs were prepared by dispersing the NPs at 10 mg/ml concentrations in pure water. Solutions were sonicated for 15 min at 35 kHz (Elma Transsonic 460/H, Germany) separately for dispersing the particles. Then, the solutions were sterilized using a 0.20-μm pore-sized sterile filter. Standard solutions were prepared from stock solutions using a culture medium. All stock and standard solutions were freshly prepared each day. FEI Tecnai G2 Spirit Bio (TWIN) High Contrast Transmission Electron Microscope (Hillsboro, USA) was used to evaluate the shape and size of ZnO and CuO NPs. For a 1-day drying process, nanoparticle solutions were dropped on a copper grid covered with 200 mesh carbon film. Then, transmission electron microscope (TEM) images of these nanoparticles were obtained at 120 kV.
Cell Culture
The human neuroblastoma cell line was purchased from the American Type Culture Collection (ATCC, USA). The cell line was cultured in 10% fetal calf serum-rich DMEM: F-12 (Ham’s) medium supplemented with 1% L-glutamine and 1% penicillin–streptomycin at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air in 75 cm2 plastic culture flasks. For all methods tested, dissociated cells were seeded in 96-well culture plates. To allow adherence and proliferation, cells were incubated for 24 h at 37 °C in a 5% CO2 humidified atmosphere. When the cultures reached 95% confluence, they were used in the assays as explained below.
Cytotoxicity Studies
For cytotoxicity studies, medium containing ZnO (0.1 to 800 μg/ml) or CuO NPs (0.01 to 800 μg/ml) were added to the wells. Then, the cell cultures were incubated for 24, 48, 72, or 96 h at 37 °C. Potential mitochondrial and lysosomal cytotoxic effects were determined with MTT and NR assays described by Mosmann [27] and Borenfreund and Puerner [28], respectively. For combined NPs, 24- and 48-h incubation times were chosen for MTT and NR assays, respectively. Protective effects of DTPA, NAC, or taurine were also evaluated in this model system.
Determination of Reactive Oxygen Species
To determine the possible formation of reactive oxygen species (ROS), a method described by Cathcart et al. [29] was applied to cell cultures using a fluorescent probe, DCFH-DA. For this purpose, SH-SY5Y cells were exposed to ZnO (100, 400, 800 μg/ml) or CuO NPs (100, 400, 800 μg/ml) alone or as a mixture (100:100, 400:400, 800:800 μg/ml) in 96-well clear bottom black plates and incubated for 24 h. Then, DCFH-DA solution (10 µM) was added to each well, and cell cultures were incubated in the dark at 37 °C for 45 min. Tert-butyl hydroperoxide (TBHP) (15 µM) was used as a positive control. Fluorescence was measured at 485 nm excitation and 535 nm emission by using a fluorescent microplate reader (SpectraMax, San Jose, USA). The percentage of control fluorescence was calculated for each result.
Mitochondrial Membrane Potential
Mitochondrial membrane potential (MMP) was determined using a cationic dye, JC-10 [30,31,32]. Cells in 96-well clear bottom black plates were exposed to ZnO (100, 400, 800 μg/ml) or CuO NPs (100, 400, 800 μg/ml) alone or as a mixture (100:100, 400:400, 800:800 μg/ml) for 24 h. Then, the cells were incubated with JC-10 dye (1 µM) in the dark at 37 °C for 20 min. FCCP (1 µM) was used as a positive control for depolarizing mitochondrial membrane potential. Red and green fluorescence of cells were measured at 540 nm excitation, 590 nm emission, and at 490 nm excitation, 525 nm emission, respectively, by using a fluorescent microplate reader (SpectraMax, San Jose, USA). The red/green fluorescence ratio was calculated to obtain change of mitochondrial membrane potential. Results were given as a percentage of the control.
Apoptosis and Intracellular Calcium Levels
Preparation of Cells for Apoptosis and Intracellular Calcium Levels
SH-SY5Y cells were seeded in 96-well clear bottom black plates and were incubated for 24 h at 37 °C in a 5% CO2 humidified atmosphere to allow adherence and proliferation. Then, the cell cultures were exposed to 100 and 800 µg/ml concentrations of ZnO, and CuO NPs alone or as a mixture following preincubation of cells with protective agents for 4 h. Possible protective effects of DTPA (5 μg/ml), NAC (1 mM), and taurine (1 mM) were evaluated against 100 µg/ml concentrations of ZnO and/or CuO NPs. Details of apoptosis and intracellular calcium levels assays were described below:
Apoptosis
To determine cellular apoptosis, ApoOne Homogeneous Caspase-3/7 Assay Kit (Promega, Wisconsin, USA) was used. The kit procedure was applied to the cell cultures as described. Then, the plates were incubated in the dark at room temperature for 30 min. Fluorescence levels were determined at 485 nm excitation and 528 nm emission wavelength. These levels were compared with control fluorescence levels for the presentation of results.
Intracellular Calcium Levels
A Fluoforte Calcium Assay Kit (Enzo Life Sciences, Farmingdale, USA) was used to evaluate intracellular calcium levels. Following the incubation of cell cultures with NPs, Fluoforte dye was added to each well, and plates were incubated in a dark place at 37 °C for 45 min. Fluorescence was measured at an excitation of 490 nm and an emission of 525 nm.
Statistical Analysis
Mann–Whitney U test was employed to calculate the statistical significance between control and treated groups. A p-value < 0.05 was considered to be statistically significant. All data were expressed as mean ± standard deviation, and at least three separate experiments were performed for each method.
Results
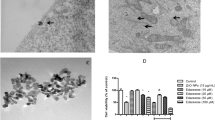
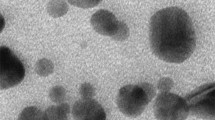
TEM Images of Zinc Oxide and Copper Oxide Nanoparticles
Transmission electron microscope images of ZnO and CuO NPs show sizes and agglomeration or aggregation states of stock solutions of these NPs (Fig. 1). Dimensions of both nanoparticles were appropriate to the sizes specified on the packages (ZnO < 100 nm, CuO < 50 nm).
Cytotoxicity
Mitochondrial Cytotoxicity
MTT results of ZnO NPs are represented in Fig. 2A. Slight decreases in cell survival were noted following 24-, 72-, or 96-h incubations. However, no cytotoxic effects were observed at 48 h. Cell viability was ≤ 57% following 24-h incubation at 200 and 400 μg/ml. Similarly, following incubation with ZnO NPs for 72 or 96 h, cell viability was determined as 54.06 and 59.62% at 800 μg/ml, respectively.
The effect of ZnO NPs on SH-SY5Y cell survival following 24, 48, 72, and 96 h. MTT (A) and NR (B) assays were used to determine survival%. For each concentration in each plate, values are the mean ± S.D. of three to four separate experiments assessed in four or eight replicates. *n = 12, #n = 9, +n = 13. ap < 0.04 vs. control, bp < 0.002 vs. control, cp < 0.02 vs. control
There was a slight dose-dependent decrease in percent survival following 24-h incubation of SH-SY5Y cells with various concentrations of CuO NPs (Fig. 3A). The concentrations of CuO NPs which caused < 50% decrease in cell viabilities were 800 μg/ml, 200 and 800 μg/ml, 400 and 800 μg/ml, and 400 μg/ml for 24, 48, 72, and 96 h, respectively.
The effect of CuO NPs on SH-SY5Y cell survival following 24, 48, 72, and 96 h. MTT (A) and NR (B) assays were used to determine survival%. For each concentration in each plate, values are the mean ± S.D. of three to five separate experiments assessed in four or eight replicates. *n = 12, #n = 8, +n = 13. ap < 0.04 vs. control, bp < 0.002 vs. control, cp <0.02 vs. control
When the cells were exposed to the mixture of ZnO: CuO NPs, the highest decreases in cell viability were measured following incubation with 12.5:12.5 and 800:800 μg/ml. The cell viability levels were determined as 59.62% and 54.93%, respectively, for those concentrations (Fig. 4).
The effect of ZnO:CuO NPs on SH-SY5Y cell survival. MTT (A) and NR (B) assays were used to determine survival%. For each concentration in each plate, values are the mean ± S.D. of three separate experiments assessed in four or eight replicates. *n = 12, +n = 8. ap < 0.002 vs. control, bp < 0.02 vs. control
Slight protective effects were obtained following preincubation of cells with DTPA, NAC, or taurine against ZnO-, CuO-, or mixture-induced cytotoxicity (Fig. 5).
The protective effects of two different concentrations of DTPA, NAC, and taurine on individual and combined ZnO, CuO NPs-induced cytotoxicity in SH-SY5Y cells. The cells were preincubated with protective agents for 4 h before addition of 100 μg/ml NPs for 24 h. MTT assay was used to determine survival%. For each concentration in each plate, values are the mean ± S.D. of three to four separate experiments assessed in four or ten replicates. *n = 8, +n = 10, #n = 9. ap < 0.002 vs. control, bp < 0.05 vs. ZnO:CuO (100:100)
Lysosomal Cytotoxicity
Neutral red assay results of ZnO NPs were shown in Fig. 2B. No cytotoxic effects were observed in SH-SY5Y cells following 24-h incubation with these NPs. There were slight fluctuations in cell viability following 48-h incubation, and the lowest cell survival percent was determined as 61.32% at 800 µg/ml of ZnO NPs. A general 30% decline was observed in the survival percent of cells following 72 h of incubation, and the lowest values determined were 64.45, 61.97, and 63.07 at 12.5, 50, and 400 µg/ml of ZnO NPs, respectively. Cell viability was < 70% at concentrations of 0.5–12.5 µg/ml and 100 and 800 µg/ml following 96-h incubation.
Results of NR assay in the presence of CuO NPs showed that (Fig. 3B) only the highest concentrations of these NPs caused marked decreases (nearly 40%) in cell survival at all incubation times especially following 96-h incubation. The determined percent survival at 96 h was 22.38%.
Neutral red assay results of a mixture of ZnO and CuO NPs were given in Fig. 4. Following 48 of hours incubation, < 70% cell survival was noted only with 100:100 or 800:800 µg/ml of ZnO:CuO NPs.
Protective effects of DTPA (2.5 and 5 µg/ml), NAC (0.5 and 1 mM), or taurine (0.5 and 1 mM) determined with NR assay were shown in Fig. 6. Preincubation (4 h) of cells with two different concentrations of these agents had marked protective effects on cell viability. Only a higher concentration of taurine (1 mM) did not have any effects on cytotoxicity induced by NP’s mixture. A comparison of both NPs’s cytotoxicity assay results was given in Fig. 7.
The protective effects of two different concentrations of DTPA, NAC, and taurine on individual and combined ZnO, CuO NPs-induced cytotoxicity in SH-SY5Y cells. The cells were preincubated with protective agents for 4 h before addition of 800 μg/ml NPs for 48 h. NR assay was used to determine survival%. For each concentration in each plate, values are the mean ± S.D. of three to four separate experiments assessed in four or nine replicates. *n = 9, +n = 8. ap < 0.02 vs. control, bp < 0.05 vs. ZnO, cp < 0.02 vs. ZnO:CuO, dp < 0.02 vs. ZnO, ep < 0.02 vs. CuO, fp < 0.05 vs. control, gp < 0.01 vs. ZnO, hp < 0.01 vs. ZnO:CuO, kp < 0.05 vs. Tau, mp < 0.05 vs. CuO
Reactive Oxygen Species Generation
DCFH-DA analysis results were shown in Fig. 8. In this experiment, tert-butyl hydroperoxide (TBHP) (15 µM) was used as a positive control. However, no increase was noted in fluorescence percent following incubation with this agent in the present model system. Similarly, ZnO NPs did not induce ROS production. We observed that only the highest concentration (800 µg/ml) of CuO NPs and all the concentrations of mixture caused increases in fluorescence percent.
The effects of individual and combined ZnO, CuO NPs on reactive oxygen species generation in SH-SY5Y cells following incubation for 24 h. For each concentration in each plate, values are the mean ± S.D. of three to four separate experiments performed in four or eight replicates. There is no statistically significance when the results compared with control or TBHP
Mitochondrial Membrane Potential
In mitochondrial membrane potential assays, FCCP was used as a positive control. In this model system, only higher concentration (5 µM) of FCCP decreased MMP levels. DMSO was used as a solvent, and it has no effect on the SH-SY5Y cell culture. A dose-dependent decline was observed in MMP levels following the incubation of cells with ZnO NPs for 24 h. Slight decreases were noted as 92.96 and 97.15% for 100 and 800 µg/ml concentrations of CuO NPs and 97.43 and 98.53% for 100:100 and 400:400 µg/ml ZnO: CuO NPs, respectively (Fig. 9). There is no statistical significance when the results compared with control, DMSO control, or FCCP.
Mitochondrial membrane potential changes in SH-SY5Y cells following incubation with individual and combined ZnO, CuO NPs for 24 h. For each concentration in each plate, values are the mean ± S.D. of three separate experiments performed in four or eight replicates. There is no statistical significance when the results compared with control, DMSO control, or FCCP
Apoptosis
Caspase 3/7 levels were increased following incubation of SH-SY5Y cells with two different concentrations of ZnO and/or CuO NPs, and all treatments with DTPA for 24 h. However, following preincubation of cells with NAC or taurine in the presence or absence of ZnO, CuO, or mixture of them, decreases were noted in caspase 3/7 levels except “ZnO plus NAC” treatment (Table 1).
Intracellular Calcium Levels
Intracellular calcium levels of cells declined following 24-h exposure to ZnO and/or CuO NPs. The results of protective agents in the presence or absence of NPs also showed similar results (Table 1).
Discussion
It has been reported that information on the neurotoxic effects of NPs has been incomplete and a continual debate exists on this subject [6]. Considering either the complexity of the nervous system or unknown properties and unexplained effects of human-made NPs in the brain, investigations on the neurotoxic effects of these types of NPs are important. In the present study, using SH-SY5Y cells as a model system for neurons [33], we investigated single or mixed cytotoxic effects of human-made ZnO and CuO NPs.
In the current study, for the preparation of stock solutions of ZnO and CuO NPs, the filter sterilization method was preferred. As indicated, this method is the only option for metal as well as other hard-structured NPs (e.g., polymeric or silica-based NPs) [34]. On the other hand, characterization studies were performed with TEM following the sterilization procedure, and the sizes of both NPs were found compatible with the dimensions stated on the packages. In the literature, aggregates were determined in characterization studies of ZnO and CuO NPs, separately [35,36,37]. In the present study, agglomeration state and distributions of ZnO and CuO NPs in stock solutions were found similar to literature data.
In the present study, a wide concentration range and four different incubation periods were chosen to obtain mitochondrial and lysosomal cytotoxicity profiles of ZnO and CuO NPs. Then, the cytotoxic effects of these NPs as a mixture were investigated following 24 h of incubation using the same concentration range for ZnO and CuO NPs alone. Concentration ranges were chosen according to previous studies performed with these NPs [22, 36, 38,39,40].
Our results indicated that ZnO NPs caused slight decreases in the survival percent of cells at 24 h when mitochondrial cytotoxic effects were determined with MTT assay. At this time point, the lowest percent survival we noted was 53% at 200 µg/ml (Fig. 2). Similarly, cytotoxic effects of ZnO NPs in SH-SY5Y cells were also shown by Kim et al. (2015) following 24 h of incubation. As their concentration range was narrow (10–30 µg/ml), we cannot make a comparison for our higher concentrations.
Following 48 h of incubation, different from other studies, we observed that ZnO NPs caused the proliferation of cells almost at all concentrations except 100 µg/ml. This effect might be the result of the induction of defense mechanisms of cells following exposure to these NPs at this time point. However, to make an exact conclusion, measurement of oxidative stress and/or antioxidant levels (e.g., glutathione or activity of antioxidant enzymes) is required. Actually, ROS levels were determined in the present study; however, the incubation time for this assay chosen was 24 h (Fig. 8). Increases in cell proliferation were also shown at ≤ 3 µg/ml of ZnO NPs on human umbilical vein endothelial cells at 5 days by Tsou et al. [41]. In addition, Pan et al. [26] reported that ZnO NPs showed protective as well as cytotoxic functions changing Zn2+ concentration and modulating expression of Zn transporter1 and Zrt/Irt-related proteins (ZIP)8 in SH-SY5Y cell culture. Therefore, the results obtained in our study might be related to this dual role of zinc.
At 72 and 96 h of incubation periods, the lowest percent survival values we determined as 54 and 59%, respectively, only at 800 µg/ml. Our MTT assay results were found different from, for instance, the results of Valdiglesias et al. [23] for these time points. Differences determined from other studies might be due to several factors including sizes and preparation of NP solutions as well as types of assays used.
Considering the lysosomal cytotoxicity results of the current study with ZnO NPs, we showed that these NPs caused slight decreases in cell survival percent, especially at 72 and 96 h of incubation. The lowest percent of survival determined was 60.44% at 1 µg/ml concentration following 96 h of incubation. These results were different from the data of Valdiglesias et al. [23]. They found that ZnO NPs caused a dose- and time-dependent lysosomal cytotoxicity in SH-SY5Y cells (≥ 25 µg/ml of ZnO NPs for 24 and 48 h).
In the present study, it has been shown that slight decreases in survival percent of cells in the presence of ZnO NPs were more noticeable, in general, with NR assay except 24 h compared to MTT assay (Fig. 7). It has been reported that ZnO NPs showed high solubility in acidic environments like lysosomes with pH 5.2 [4, 42], and release zinc ions [4]. Numerous studies also indicated that the toxicity of ZnO NPs is related to released zinc ions [13, 43,44,45,46]. Although we did not measure Zn2+ ion concentrations in this study, it could be suggested that more pronounced induction of lysosomal cytotoxicity by ZnO NPs might be related to zinc ion release due to the acidic environment of this organelle.
Regarding our MTT results with CuO NPs, dose-dependent slight decreases in cell viability were observed especially at 24 and 48 h. These results were similar to data of Jang et al. [25] and Chen et al. [21]. Lysosomal cytotoxicity results of CuO NPs showed more fluctuations nearly at all concentrations and incubation times. Only at 96 h with the highest concentration of CuO NPs, the lowest survival percent was determined as 22%. On the other hand, we noted that CuO NPs caused increases in cell survival percent at 24 h nearly with all concentrations tested (0.5 to 50 µg/ml and at 400 µg/ml) when the NR test was used. Increases were also observed nearly at all concentrations between 12.5 and 200 µg/ml of CuO NPs following 72 and 96 h of incubation.
On the other hand, current results of two cytotoxicity tests in the presence of CuO NPs showed a different profile: With CuO NPs, mitochondrial cytotoxicity was more pronounced especially at 24 and 48 h as well as ≥ 1 µg/ml at 72 h. Hsueh et al. [47] reported that CuO NPs are more soluble in acidic pH. They suggested that CuO NPs showed higher toxicity due to the increasing release of Cu2+ ions. As mitochondrial pH levels are around 8 [48, 49], our above-mentioned explanation for lysosomal cytotoxicity of ZnO NPs cannot be applied to this situation. Therefore, further studies are needed to explain these effects.
Humans and all other organisms are exposed to various man-made nanoparticles and mixtures of them via several routes. Hence, the determination of their toxic effects, especially as a mixture is an important research area. In several studies, the toxic effects of ZnO and CuO NPs’ mixture on plants [50] and aquatic organisms [46, 51] have been shown. This is the first study investigating the cytotoxic effects of ZnO and CuO NPs’ mixture in human cell culture systems.
Preincubation of cells with two different concentrations of DTPA, NAC, or taurine mildly protected cells from ZnO-, CuO-, and the mixture of these NP-induced slight cytotoxicity when the MTT test was used. Protective effects obtained by these agents were much more pronounced with the NR assay. Overall, this protection was found to be particularly significant in the presence of 0.5 mM NAC against cytotoxicity caused by both CuO NPs- and mixture of these NPs. Considering the protection provided by DTPA, it could be suggested that metal ions might be released from ZnO and/or CuO NPs. Since we did not measure metal ion release in the present model system, we cannot draw an exact conclusion on this subject. Kim et al. [9] also showed that ZnO NP-induced ROS production was suppressed by an iron-specific chelator, deferoxamine. They [9] indicated that this finding is important suggesting future use of metal chelators in the therapy or prevention of different diseases caused by metal-associated NPs [9, 13, 52]. Protective effects obtained with a glutathione precursor NAC [24] in the present study suggest that oxidative stress might be involved in the cytotoxicity mechanisms of these NPs. Supporting our results, Kim et al. (2015) also showed the protective effects of NAC against ZnO NPs-induced cytotoxicity in SH-SY5Y cells. With its antioxidant properties [53,54,55,56] as well as the capacity to improve mitochondrial function and inhibit the generation of ROS [55,56,57,58], protection obtained in the presence of taurine also supports oxidative stress involvement in the cytotoxicity mechanism of these NPs in the present model system. In agreement with other studies, all our findings suggest that oxidative stress has an important role in ZnO and CuO NPs-induced cytotoxic effects. Additionally, this protection was also effective against the cytotoxic effect of the mixture of these NPs.
Oxidative stress and ROS have been linked to cell death of neurons in many neurodegenerative conditions [59]. In order to determine the role of possible ROS production in the cytotoxicity mechanisms of these NPs, ROS levels were measured using DCFH-DA assay. In the present study, ROS levels were slightly increased only with the highest concentration (800 µg/ml) of CuO NPs and all concentrations (100:100, 400:400, and 800:800 µg/ml) of the mixture of NPs. These findings could be considered supportive evidence of the protection obtained with NAC and taurine against the cytotoxic effects of these NPs. Similar to our results, increases in ROS levels by CuO NPs were also shown in various cell cultures. For example, Siddiqui et al. [60] reported that 22-nm-sized CuO NPs (2–10 µg/ml) induced the intracellular production of ROS dose-dependently in human hepatocarcinoma cells following 24 h of incubation.
On the other hand, it has been reported that DCF formation was affected in the presence of reducing agents [61]. Therefore, in the present study, due to an interaction possibility, DTPA, NAC, or taurine were not used in DCFH-DA analysis.
Mitochondrial membrane potential and apoptosis levels were also evaluated to understand the underlying mechanisms of cytotoxic effects of ZnO and CuO NPs in SH-SY5Y cells. In this study, the mitochondrial membrane potential of SH-SY5Y cells was found lower than the control group following exposure to the highest concentration (800 µg/ml) of ZnO NPs, and the lowest (100 µg/ ml) concentration of CuO NPs. Actually, decreases were found slight and determined as 10% and 8% for 800 µg/ml ZnO and 100 µg/ml CuO NPs, respectively. Similarly, decreases in MMP levels were reported following ZnO NPs exposure in various cell cultures including HepG2 [62], C3A [63], and retinal ganglion cells [64]. However, Valdiglesias et al. [23] showed that ZnO NPs caused an increase in MMP levels at 20, 30, and 40 µg/ml concentrations following 3 h, and at 30 and 40 µg/ml concentrations following 6 h of incubation in SH-SY5Y cells. On the other hand, similar to our results, Siddiqui et al. [60] reported that CuO NPs had MMP-decreasing effects in HepG2 cells in conditions different from the present study.
It has been known that mitochondrial membrane potential decreases during apoptosis [60, 65]. Caspase 3/7 activity which shows the status of apoptosis was usually increased following exposure to nanoparticles. Likewise, in the present study, these activities were induced following 24 h of exposure to ZnO and CuO NPs at concentrations we have determined according to the results of cytotoxicity tests. In the presence of the mixture of ZnO and CuO NPs, caspase 3/7 activities were also increased. Similar results were also obtained in various cell types including SH-SY5Y cells. For instance, Kim et al. [24] reported that total apoptotic cells (as evaluated alterations of Annexin V and caspase-3/7 activity) were significantly increased at 10–15 µg/mL concentrations of ZnO NPs. Additionally, Song et al. [66] showed that caspase 3/7 activity of astrocyte cells was increased significantly following exposure to 3 and 10 µg/ml concentrations of ZnO NPs at 12 h. It has been reported that higher concentrations of ZnO NPs (15–30 µg/ml) had similar effects on apoptosis in human bone marrow-derived mesenchymal stem cells following 12 and 24 h of incubation [9]. Siddiqui et al. [60] indicated that 10 µg/ml concentration of CuO NPs significantly upregulated apoptotic genes bax and caspase-3 in HepG2 cells following 4 h of incubation. However, in several studies, it has been reported [67, 68] that similar concentrations of the same nanoparticles did not have the same effects on caspase 3/7-mediated apoptosis. This might be due to the differences in culture conditions or cellular environment [66].
In the current study, DTPA alone caused an increase in caspase 3/7 levels; however, it did not reduce ZnO and CuO NP-induced caspase 3/7 levels. On the other hand, we found that NAC or taurine had protective effects. Apoptosis-decreasing effects of NAC or taurine in cell culture systems were also reported in a few studies [69, 70]. For example, Ostrovsky et al. (2009) indicated that due to the ROS quenching effects of NAC, U87 cells protected against ZnO NPs-induced apoptosis.
It has been reported that intracellular calcium levels increased in the presence of nanoparticles [71,72,73]. However, there was not any study on the effects of CuO NPs on calcium levels in cell culture systems. In the present study, there were decreases in intracellular calcium levels of SH-SY5Y cells following 24 h of incubation of cells with ZnO and CuO NPs individually or as a mixture. DTPA, NAC, or taurine had also similar effects in these levels.
Conclusions
In conclusion, present results indicated that ZnO or/and CuO NPs have slight mitochondrial and lysosomal cytotoxic effects in human neuroblastoma cell lines. Protection obtained against these effects with DTPA suggests that Zn2+ and Cu2+ ions might have a role in the NPs-induced cytotoxicity. On the other hand, the protective effects provided by NAC and taurine suggest that oxidative stress plays a role in the cytotoxicity mechanism of these NPs. Alterations in the levels of ROS and MMP were also shown in this model system. In addition to protective effects obtained with NAC and taurine in cytotoxicity assays, our results showing ROS inducing potential of CuO NP alone and the mixture of ZnO and CuO NPs might be considered as supportive evidence to the role of ROS in cytotoxicity mechanisms of these NPs (Fig. 10). DTPA, NAC, or taurine had protective effects against apoptosis induced by these NPs. We believe that the data obtained in the current study provide new knowledge about either alone or in mixtures cytotoxic effects of ZnO and CuO NPs. However, additional studies are needed to understand the exact toxicity mechanisms of these NPs.
ZnO and CuO NPs induced slight cytotoxic effects either individually or as a mixture in SH-SY5Y cells. Protection noted in the presence of DTPA, NAC, or taurine against cytotoxicity of these NPs. ROS inducing effect of CuO NPs and mixture of two NPs suggest that oxidative stress might be involved in cytotoxicity mechanisms of these NPs
References
Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71(7):1308–1316
Deng X, Luan Q, Chen W, Wang Y, Wu M, Zhang H, Jiao Z (2009) Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 20(11):115101
Iqbal A, Ahmad I, Khalid MH, Nawaz MS, Gan SH, Kamal MA (2013) Nanoneurotoxicity to nanoneuroprotection using biological and computational approaches. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 31(3):256–284
Liu J, Kang Y, Yin S, Song B, Wei L, Chen L, Shao L (2017) Zinc oxide nanoparticles induce toxic responses in human neuroblastoma SHSY5Y cells in a size-dependent manner. Int J Nanomed 12:8085–8099
Feron VJ, Groten JP (2002) Toxicological evaluation of chemical mixtures. Food Chem Toxicol 40(6):825–839
Bencsik A, Lestaevel P, Guseva Canu I (2018) Nano- and neurotoxicology: an emerging discipline. Prog Neurobiol 160:45–63
Klaassen CD, Casarett LJ, Doull J (2013) Casarett and Doull’s toxicology: the basic science of poisons, 8th edn. McGraw-Hill Education / Medical, New York
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87(7):1181–1200
Kim DY, Kim JH, Lee JC, Won MH, Yang SR, Kim HC, Wie MB (2019) Zinc oxide nanoparticles exhibit both cyclooxygenase- and lipoxygenase-mediated apoptosis in human bone marrow-derived mesenchymal stem cells. Toxicol Res 35(1):83–91
Bengalli R, Colantuoni A, Perelshtein I, Gedanken A, Collini M, Mantecca P, Fiandra L (2021) In vitro skin toxicity of CuO and ZnO nanoparticles: application in the safety assessment of antimicrobial coated textiles. NanoImpact 21:100282
Arora S, Rajwade JM, Paknikar KM (2012) Nanotoxicology and in vitro studies: the need of the hour. Toxicol Appl Pharmacol 258(2):151–165
Gawande MB, Goswami A, Felpin F-X, Asefa T, Huang X, Silva R, Zou X, Zboril R, Varma RS (2016) Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev 116(6):3722–3811
Liu G, Men P, Perry G, Smith MA (2010) Nanoparticle and iron chelators as a potential novel Alzheimer therapy. Methods Mol Biol 610:123–144
Zanoni I, Crosera M, Ortelli S, Blosi M, Adami G, Larese F, Costa A (2019) CuO nanoparticle penetration through intact and damaged human skin. New J Chem 43:17033–17039
Adeyemi JA, Machado ART, Ogunjimi AT, Alberici LC, Antunes LMG, Barbosa F Jr (2020) Cytotoxicity, mutagenicity, oxidative stress and mitochondrial impairment in human hepatoma (HepG2) cells exposed to copper oxide, copper-iron oxide and carbon nanoparticles. Ecotoxicol Environ Saf 189:109982
Zerboni A, Bengalli R, Baeri G, Fiandra L, Catelani T, Mantecca P (2019) Mixture effects of diesel exhaust and metal oxide nanoparticles in human lung A549 cells. Nanomaterials (Basel) 9(9):1302
Parsai T, Kumar A (2021) Weight-of-evidence process for assessing human health risk of mixture of metal oxide nanoparticles and corresponding ions in aquatic matrices. Chemosphere 263:128289
Wu J, Ding T, Sun J (2013) Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology 34:243–253
Song B, Liu J, Feng X, Wei L, Shao L (2015) A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res Lett 10:342
Valdiglesias V, Kiliç G, Costa C, Fernández-Bertólez N, Pásaro E, Teixeira JP, Laffon B (2015) Effects of iron oxide nanoparticles: cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ Mol Mutagen 56(2):125–148
Chen J, Zhu J, Cho H-H, Cui K, Li F, Zhou X, Rogers J, Wong S, Huang X (2008) Differential cytotoxicity of metal oxide nanoparticles. J Exp Nanosci 3(4):321–328
Perreault F, Pedroso Melegari S, da Henning Costa C, de Oliveira Franco Rossetto AL, Popovic R, Gerson Matias W (2012) Genotoxic effects of copper oxide nanoparticles in Neuro 2A cell cultures. Sci Total Environ 441:117–124
Valdiglesias V, Costa C, Kiliç G, Costa S, Pasaro E, Laffon B, Teixeira JP (2013) Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ Int 55:92–100
Kim JH, Jeong MS, Kim DY, Her S, Wie MB (2015) Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem Int 90:204–214
Jang S-W, Oh M-S, Yang SI, Cho E-M (2016) Gene expression profiles of human neuroblastoma cells exposed to CuO nanoparticles and Cu ions. BioChip J 10(2):140–149
Pan CY, Lin FY, Kao LS, Huang CC, Liu PS (2020) Zinc oxide nanoparticles modulate the gene expression of ZnT1 and ZIP8 to manipulate zinc homeostasis and stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. PLoS ONE 15(9):e0232729
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Borenfreund E, Puerner JA (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24(2–3):119–124
Cathcart R, Schwiers E, Ames BN (1983) Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134(1):111–116
Reers M, Smith TW, Chen LB (1991) J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 30(18):4480–4486
Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Chen LB (1991) Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A 88(9):3671–3675
Donaghy L, Kraffe E, Le Goic N, Lambert C, Volety AK, Soudant P (2012) Reactive oxygen species in unstimulated hemocytes of the pacific oyster Crassostrea gigas: a mitochondrial involvement. PLoS ONE 7(10):e46594
Xie HR, Hu LS, Li GY (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J (Engl) 123(8):1086–1092
Desai N (2012) Challenges in development of nanoparticle-based therapeutics. AAPS J 14(2):282–295
Kleinsasser J (2010) Zinc oxide nanoparticles induce photocatalytic cell death in human head and neck squamous cell carcinoma cell lines in vitro. Int J Oncol 37:6
Hackenberg S, Scherzed A, Kessler M, Froelich K, Ginzkey C, Koehler C, Burghartz M, Hagen R, Kleinsasser N (2010) Zinc oxide nanoparticles induce photocatalytic cell death in human head and neck squamous cell carcinoma cell lines in vitro. Int J Oncol 37(6):1583–1590
Bulcke F, Thiel K, Dringen R (2014) Uptake and toxicity of copper oxide nanoparticles in cultured primary brain astrocytes. Nanotoxicology 8(7):775–785
Sun J, Wang S, Zhao D, Hun FH, Weng L, Liu H (2011) Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells: cytotoxicity, permeability, and inflammation of metal oxide nanoparticles. Cell Biol Toxicol 27(5):333–342
Di Bucchianico S, Fabbrizi MR, Misra SK, Valsami-Jones E, Berhanu D, Reip P, Bergamaschi E, Migliore L (2013) Multiple cytotoxic and genotoxic effects induced in vitro by differently shaped copper oxide nanomaterials. Mutagenesis 28(3):287–299
Uzar NK, Abudayyak M, Akcay N, Algun G, Ozhan G (2015) Zinc oxide nanoparticles induced cyto- and genotoxicity in kidney epithelial cells. Toxicol Mech Methods 25(4):334–339
Tsou TC, Yeh SC, Tsai FY, Lin HJ, Cheng TJ, Chao HR, Tai LA (2010) Zinc oxide particles induce inflammatory responses in vascular endothelial cells via NF-κB signaling. J Hazard Mater 183(1–3):182–188
Muller KH, Kulkarni J, Motskin M, Goode A, Winship P, Skepper JN, Ryan MP, Porter AE (2010) pH-dependent toxicity of high aspect ratio ZnO nanowires in macrophages due to intracellular dissolution. ACS Nano 4(11):6767–6779
Zhao J, Xu L, Zhang T, Ren G, Yang Z (2009) Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology 30(2):220–230
Chen P, Powell BA, Mortimer M, Ke PC (2012) Adaptive interactions between zinc oxide nanoparticles and Chlorella sp. Environ Sci Technol 46(21):12178–12185
Bhuvaneshwari M, Iswarya V, Archanaa S, Madhu GM, Kumar GK, Nagarajan R, Chandrasekaran N, Mukherjee A (2015) Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aquat Toxicol 162:29–38
Ye N, Wang Z, Fang H, Wang S, Zhang F (2017) Combined ecotoxicity of binary zinc oxide and copper oxide nanoparticles to Scenedesmus obliquus. J Environ Sci Health A Tox Hazard Subst Environ Eng 52(6):555–560
Hsueh Y-H, Tsai P-H, Lin K-S (2017) pH-dependent antimicrobial properties of copper oxide nanoparticles in Staphylococcus aureus. Int J Mol Sci 18(4):793
Casey JR, Grinstein S, Orlowski J (2010) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11(1):50–61
Sarkar AR, Heo CH, Xu L, Lee HW, Si HY, Byun JW, Kim HM (2016) A ratiometric two-photon probe for quantitative imaging of mitochondrial pH values. Chem Sci 7(1):766–773
Dimkpa CO, McLean JE, Britt DW, Anderson AJ (2015) Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology 24(1):119–129
Xia J, Zhao HZ, Lu GH (2013) Effects of selected metal oxide nanoparticles on multiple biomarkers in Carassius auratus. Biomed Environ Sci 26(9):742–749
Wang N, Jin X, Guo D, Tong G, Zhu X (2017) Iron chelation nanoparticles with delayed saturation as an effective therapy for Parkinson disease. Biomacromol 18(2):461–474
Aruoma OI, Halliwell B, Hoey BM, Butler J (1989) The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6(6):593–597
Chen G, Nan C, Tian J, Jean-Charles P, Li Y, Weissbach H, Huang XP (2012) Protective effects of taurine against oxidative stress in the heart of MsrA knockout mice. J Cell Biochem 113(11):3559–3566
Ripps H, Shen W (2012) Review: taurine: a “very essential” amino acid. Mol Vis 18:2673–2686
Marcinkiewicz J, Kontny E (2014) Taurine and inflammatory diseases. Amino Acids 46(1):7–20
Schaffer SW, Azuma J, Mozaffari M (2009) Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87(2):91–99
Jong CJ, Azuma J, Schaffer S (2012) Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 42(6):2223–2232
Sanvicens N, Gomez-Vicente V, Messeguer A, Cotter TG (2006) The radical scavenger CR-6 protects SH-SY5Y neuroblastoma cells from oxidative stress-induced apoptosis: effect on survival pathways. J Neurochem 98(3):735–747
Siddiqui MA, Alhadlaq HA, Ahmad J, Al-Khedhairy AA, Musarrat J, Ahamed M (2013) Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS One 8(8):e69534
Rota C, Fann YC, Mason RP (1999) Phenoxyl free radical formation during the oxidation of the fluorescent dye 2′,7′-dichlorofluorescein by horseradish peroxidase. Possible consequences for oxidative stress measurements. J Biol Chem 274(40):28161–28168
Yuan L, Wang Y, Wang J, Xiao H, Liu X (2014) Additive effect of zinc oxide nanoparticles and isoorientin on apoptosis in human hepatoma cell line. Toxicol Lett 225(2):294–304
Filippi C, Pryde A, Cowan P, Lee T, Hayes P, Donaldson K, Plevris J, Stone V (2015) Toxicology of ZnO and TiO2 nanoparticles on hepatocytes: impact on metabolism and bioenergetics. Nanotoxicology 9(1):126–134
Guo D, Bi H, Liu B, Wu Q, Wang D, Cui Y (2013) Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol In Vitro 27(2):731–738
Sharma V, Anderson D, Dhawan A (2012) Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 17(8):852–870
Song WJ, Jeong MS, Choi DM, Kim KN, Wie MB (2019) Zinc oxide nanoparticles induce autophagy and apoptosis via oxidative injury and pro-inflammatory cytokines in primary astrocyte cultures. Nanomaterials (Basel) 9:7
Wang J, Deng X, Zhang F, Chen D, Ding W (2014) ZnO nanoparticle-induced oxidative stress triggers apoptosis by activating JNK signaling pathway in cultured primary astrocytes. Nanoscale Res Lett 9(1):117
Sudhakaran S, Athira SS, Mohanan PV (2019) Zinc oxide nanoparticle induced neurotoxic potential upon interaction with primary astrocytes. Neurotoxicology 73:213–227
Ostrovsky S, Kazimirsky G, Gedanken A, Brodie C (2009) Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res 2(11):882–890
Niu X, Zheng S, Liu H, Li S (2018) Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol Med Rep 18(5):4516–4522
Zhang J, Song W, Guo J, Zhang J, Sun Z, Ding F, Gao M (2012) Toxic effect of different ZnO particles on mouse alveolar macrophages. J Hazard Mater 219–220:148–155
Guo D, Bi H, Wang D, Wu Q (2013) Zinc oxide nanoparticles decrease the expression and activity of plasma membrane calcium ATPase, disrupt the intracellular calcium homeostasis in rat retinal ganglion cells. Int J Biochem Cell Biol 45(8):1849–1859
Wang D, Guo D, Bi H, Wu Q, Tian Q, Du Y (2013) Zinc oxide nanoparticles inhibit Ca2+-ATPase expression in human lens epithelial cells under UVB irradiation. Toxicol In Vitro 27(8):2117–2126
Acknowledgements
TEM images of nanoparticles were obtained at the Middle East Technical University (METU) Central Laboratory.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by the Hacettepe University Research Foundation Projects (Project Number: 014D12301001-830 and Project Number: THD-2017–14012).
Author information
Authors and Affiliations
Contributions
A.G. and D.P. wrote the main manuscript text, A.G. designed and D.P. prepared figures and table. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paslı, D., Gürbay, A. Assessment of Protective Effects of DTPA, NAC, and Taurine on Possible Cytotoxicity Induced by Individual and Combined Zinc Oxide and Copper Oxide Nanoparticles in SH-SY5Y Cells. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04161-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04161-0