Abstract
Rutin, a natural bioflavonoid compound, is one of the best-known antioxidants. This study aimed to investigate the protective effect of rutin-loaded chitosan alginate nanoparticles (RCA NPs) against lead (Pb)-induced oxidative stress in two different broiler breeds. A total number of 240 chicks from Cobb (CB) and Arbor Acres (AR) breeds were randomly allocated into 4 groups/breed. The 1st group received standard basal diet (SD) and drinking water (DW) while the 2nd group received SD and Pb-incorporated DW (350 mg/L). The 3rd group treated with both rutin-supplemented SD (50 mg/kg feed), and DW contain Pb (350 mg/L). Finally, the 4th group administered RCA NPs-supplemented SD (50 mg/kg feed) and Pb-incorporated DW (350 mg/L). On the 40th day of experiment, broilers weighed, and blood samples collected for biochemical and hematological analysis then slaughtered. Economic efficiency, growth performance, and oxidative stress biomarkers were evaluated. Gene expression level of growth-associated genes as insulin-like growth factor-I (IGF-1) and histopathological changes were assessed in liver and intestinal tissue of both breeds. Our results revealed that Pb-treated birds exhibited the lowest average body weight gain (BWG) and economic efficiency measures in both breeds while RCA NPs-treated groups revealed enhanced growth and economic performance. Furthermore, diet supplementation with RCA NPs considerably enhanced the antioxidant enzymes activity and expression of growth-associated genes than groups treated with rutin alone specifically in AR breed. In conclusion, RCA NPs supplementation could be a promising nanoformulation in poultry production through enhancing the antioxidant capacity and bioavailability of rutin.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Broilers contribute significantly to human protein needs, because of their shorter life cycle, less capital and land requirements [1]. A major objective of the poultry industry is to continually improve the genetic potential of broiler strains to meet the high-quality with low-cost protein needs of the global population [2]. While producers are seeking a healthy bird with high sale in the shortest possible timeframe, consumers desire tenderness and carcass quality [3]. However, exposure of birds to oxidative stress is a leading non-microbial factor that diminishes meat quality by generating excessive free radicals which lead to cell membrane and mitochondrial damage through lipid peroxidation [4]. Moreover, these stressors are known to depress metabolic rates, retard growth, and increase susceptibility to diseases and reproductive dysfunction in birds [5]. Heavy metals, as major environmental stressors, are still a problem for the poultry industry [6]. Pb is a highly toxic heavy metal that can induce various physiological and biochemical dysfunctions in humans, animals, and birds [7, 8]. Birds can get intoxicated by drinking water contaminated with Pb from old pipes or equipment contaminated with building materials [9]. According to [10], Pb could induce oxidative stress in birds that become weak, ataxic, lose their appetite, lose weight, and get anemic [11]. A chronic Pb exposure causes motor nerve degeneration and peripheral nerve loss, muscle atrophy and even trace levels retard growth and make feed inefficient, resulting in significant economic losses in poultry farms [12]. Nowadays, feed additives are used to exclude the heavy metals-induced oxidative damage, but these additives should not have any negative effects on the performance of birds or on cost-benefit analysis [13].
In recent years, phytogenic feed additives as flavonoids which are natural plant-based bioactive molecules have gained a great deal of attention due to their ability to control diseases beside their economic accessibility and negligible harmful consequences [14], also have potent anti-oxidant properties and performance-boosting effects [15]. Rutin is a flavonoid found in many plants and has numerous benefits, including anti-oxidant, anti-inflammatory, antimicrobial, and antitumor properties [16]. Rutin also commonly used in birds to boost their growth and improve their meat quality because of their widespread availability and safety [17, 18]. Unfortunately, rutin has a major disadvantage due to its poor bioavailability and stability, which may inhibit its biological activity, so it needs to be administered at higher doses to exert its antioxidant effect [19]. Emerging the field of nanotechnology offered new incites in flavonoids delivery systems through modulation of their pharmacokinetic properties which could markedly enhance their clinical potential as potent anti-oxidant agents [20]. Chitosan alginate nanoparticles considered a new natural polymeric vehicle that could improve bioavailability, bioadhesion, targetability, and sustained release of drugs [21]. Furthermore, chitosan nanoparticles could improve young broiler chicken growth performance, production characteristics and intestinal morphology evidenced by hypertrophied villi and epithelial cells [22]. Moreover, chitosan nanoparticles can serve as a chelating agent with a strong affinity for metal ions as Pb [23]. The coupling of alginate, a natural polyanionic polysaccharide, to chitosan nanoparticles can enhance drug uptake via raising the surface charge and could serve as a promising carrier for flavonoids delivery to target tissues [24].
Therefore, this study was designed to evaluate the protective effect of rutin RCA NPs against Pb-induced oxidative stress in terms of economics, growth performance, molecular, biochemical, and histopathological changes, also to compare CB and AR broiler breeds’ responses to Pb-induced oxidative damage.
Materials and Methods
Rutin-Loaded Chitosan Alginate Nanoparticle Synthesis
Chitosan alginate nanoparticles were prepared through electrostatic gelation method with a slight modification according to [25]. In brief, chitosan (SRL Ltd., Maharashtra, India) 1% (w/v) was dissolved in diluted acetic acid 1% (v/v) then stirred with rutin (Acros Organics, New Jersey, USA) ethanolic solution (10 mg/ml) for 1 h at 500 rpm. Afterwards, Sodium tripolyphosphate (Piochem, Giza, Egypt) 0.67% (w/v) was added to the above mixture and stirring continued for 2 h. Chitosan-loaded rutin nanosuspension was centrifuged at 5000 rpm for 30 min and then stirred with sodium alginate (SRL Ltd., Maharashtra, India) 0.5% (w/v) for 30 minutes at 500 rpm. Crosslinking of chitosan-loaded rutin nanoparticles with alginate was achieved through addition of CaCl2 (0.6 M) followed by stirring for 15 min at 250 rpm; then, the resulting mixture was centrifuged at 5000 rpm for 30 min and dried at hot air oven for 12 h at 60 °C for further analysis.
Characterization of Rutin-Loaded Chitosan Alginate Nanoparticles
RCA NPs morphology was characterized through transmission electron microscope whereas a drop of diluted sample was deposited and fixed on a copper grid before analysis [26]. Dynamic light scattering (DLS) was used to assess droplet size (nm), zeta potential (mV), and polydispersity index (PDI) of RCA NPs using a Zetasizer Nano ZS (ZEN3600, Malvern Ltd., UK), and three independent measurements were taken of RCA NPs at 25 °C.
Experimental Design, Diets, and Management
Housing, management, and all birds’ related procedures were conducted according to the guidelines of Mansoura University Animal Care and Use Committee (MU-ACUC), No. (VM.R.22.11.28). A total of 240 1-day-old broiler chicks, 120 CB and 120 AR breed, were purchased from a commercial company (Mansoura Poultry Company, Mansoura, Egypt) and randomly divided into 4 groups/breed with 6 replicates/group and 5 chicks/replicate. Chicks were maintained in adequately ventilated, littered room with a density of 10 birds/m2 under uniform management, hygiene, and housing conditions. A 23L: 1D lighting program was provided on arrival then gradually decreased to 16L: 8D by day 24 until slaughter, and temperature was maintained at 33°C in the first 3 days then gradually decreased by 3°C per week until 25°C. Feed and water were provided ad libitum. All birds were vaccinated against Newcastle disease (ND) and infectious bronchitis (IB) at 7th day of age (MEVAC HB1 + H120, MEVAC Co., Egypt) then revaccinated against ND at 15th day of age (MEVAC ND, MEVAC Co., Egypt), and avian influenza at 10th day of age (MEFLUVAC, MEVAC Co., Egypt) and infectious bursal disease (IBD) at 18th day of age (UNIVAX-BD, MSD Co., USA) following the manufactures’ protocols.
Formulation of diets was based on National Research Council nutrient recommendations [27], as the feeding program consists of three phases: starter (0–14 days), grower (15–28 days), and finisher (29–40 days). Initially, birds were given a corn-soybean meal-based starter ration with 2950 Kcal.ME/Kg, 23% C.P, 2.13% C.F., and 3.35% EE from day followed by the grower ration containing 3000 Kcal.ME/Kg, 21.5% C.P., 2.4% C.F., and 4% EE. The final phase provides them with a finisher ration that contains 3050 Kcal.ME/Kg, 21% C.P., 2.44% C.F, and 4.04% EE until rearing is complete.
Chicks were allocated into 4 treatment groups for each breed as follows;
-
The 1st group (control); received SD and DW without treatment.
-
The 2nd group (Pb) as lead acetate (Tianjinzhiyuan Chemical Reagent Co., Ltd. Tianjin, China); received SD and Pb-incorporated DW (350 mg/L), according to [28].
-
The 3rd group (rutin + Pb); received both rutin-supplemented SD (50 mg/kg feed), according to [29, 30] and DW contain Pb (350 mg/L).
-
The 4th group (RCA NPs + Pb); received both RCA NPs-supplemented SD (50 mg/kg feed) and Pb-incorporated DW (350 mg/L).
Chickens were inspected daily for any disorders until time of slaughtering (40 day) and feed intake, individual BW, and feed conversion ratio per pen were recorded weekly.
Economic Efficiency
On day 40 after rearing started, all birds were sold and economic data were collected for analysis [31].
Costs of Production
Bird’s production costs were calculated based on three categories including total variable costs (TVC), total fixed costs (TFC), and total costs (TC) [32, 33].
Feed cost/ bird plus cost of supplements (rutin or RCA NPs) consumed/bird per Egyptian pound (LE) based on the market price at time of experiment calculated as feed cost, while TVC/ bird equal feed cost plus chick cost .
TFC as stated by [34, 35] consists of litter, labor, veterinary management (drugs and vaccines), water, electricity, building and equipment rent, transportation, and miscellaneous costs, plus the cost of the birds purchased [36, 37] (all costs based on market price at experiment time) [38].
Total Returns (TR) [39]
Net Profit (NP) [32, 40]
Economic Efficiency Measurements
In accordance with [37], efficiency measures were calculated as percentages of total return to total costs (TR/TC), total cost to total return (TC/TR), net profit to total cost (NP/TC), and net profit to total return (NP/TR).
Growth Performance
The average live body weight (BW) was recorded weekly; also, the average body weight gain (BWG) was measured for each replicate within groups by subtracting BW at end and start of the rearing periods. Furthermore, the average daily weight gain was also calculated (ADG= BWG/number of days) according to [41, 42]. Moreover, relative growth rate (RGR) was evaluated as ascribed by [43] where RGR = ((W2−W1)/ (0.5 (W2+W1)) ×100 as W1 = initial weight and W2 = final weight.
Feed intake was calculated at the end of each week during experiment considering the number of the dead chicks plus the number of days they fed; then, the feed conversion ratio (FRC) was calculated for each replicate within each group for each feeding phase according to the method reported by [44] whereas FCR = (feed intake / BWG).
Collection of Samples
At 40 D of age, the feed was deprived for 12 h, 3 broilers per replicate were randomly selected and weighed, and blood samples were collected immediately from the wing vein with and without anticoagulant for hematological and biochemical analysis, respectively; then, birds were slaughtered (n = 18 per group). Liver and intestinal samples were dissected and rinsed with saline and liver samples were divided into three parts. The first part was homogenized in ice-cold phosphate buffer saline (pH 7.4) then centrifuged (3000 rpm for 30 min at 4 °C) and supernatant was collected then stored at −80 °C for oxidative stress analysis. The second part stored immediately at −80 °C for molecular analysis while the third part was fixed in neutral buffer formalin (10%, pH 7.0) for histopathological examination.
Hematological and Biochemical Analysis
Hematological Examination
Blood samples with anticoagulant were used for the determination of erythrocyte count (RBCs) and hemoglobin (Hb) concentration according to [45] using an automated hematology analyzer (Sysmex KX-21N).
Biochemical Analysis
The concentrations of total protein (TP), total triglycerides (TG), total cholesterol (TCh), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C) in serum samples were measured by colorimetric methods with commercial diagnostic kits (Biosino Biotechnology and Science Inc., Beijing, China) through biochemical analyzer (Hitachi Modular System, Hitachi Ltd., Tokyo, Japan).
Growth Hormone (GH) Measurement
GH was determined in chickens’ serum samples by ELISA technique with commercially available kits (CSB-E09866Ch), (Cusabio Technology LLC, Houston, TX 77054, USA). GH concentrations were measured in serum samples according to the manufacturers’ instructions as pg/ml at 450 nm through a micro-plate ELISA reader (STAT FAX - 2100) with 625 pg/mL sensitivity and a detection limit ranged from 625 to 10000 pg/mL [46].
Assessment of Oxidative Stress and Antioxidant Parameters
Lipid peroxidation (LPO) was measured in terms of malondialdehyde (MDA) production which was evaluated following the method reported by [47]; the spectrophotometric absorbance was recorded at 535 nm; then, MDA levels were recorded as nmol/g tissue. GSH content was measured through colorimetric spectrophotometric assay reported by [48]; then, supernatant absorbance was recorded at 412 nm and expressed as mg/g tissue. Catalase (CAT) activity was assayed as reported by [49] based on the decomposition rate of H2O2 that measured at 240 nm and CAT activity was expressed as units/gm tissue.
The activity of superoxide dismutase (SOD) was assayed according to [50] based on reduction of nitro blue tetrazolium (NBT) into blue formazan and absorptance was recorded at 560 nm; SOD activity was recorded as U /g tissue. Glutathione peroxidase (GPx) activity was determined using H2O2 as substrate following the method reported by [51] since the reaction was monitored spectrophotometrically at 240 nm and GPx activity was expressed as U /g tissue.
Quantitative RT-PCR Analysis
TRIzol reagent (Thermo Fisher Scientific, USA, (15596018) was used to extract RNA from frozen liver tissues. RNA was first extracted by homogenization in TRIzol reagent. At 260 and 280 nm absorbance, Nano Photometer® spectrophotometer was used to check concentration and purity of RNA. In the next step, we synthesized cDNA from isolated RNA through Quantitect® Reverse Transcription kit (Qiagen, Germany) according to the manufacturer’s instructions. The target genes were then amplified using forward and reverse primers, their sequences and GenBank accession numbers were listed in Table 1 along with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene (internal control) for normalizing expression levels. Quantitative real-time PCR (qRT-PCR) was used to measure the expressions of IGF-I, IGF-II, GHR, and IGFBP genes in liver tissues using a Rotor-Gene Q instrument and QuantiTect® SYBR® Green PCR kit (Qiagen, Germany). The amplification conditions were 95 °C for ten minutes, followed by 40 cycles of 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. As described by [52], each gene expression pattern was calculated through the comparative 2-ΔΔCt method.
Histopathological Examination
Samples from the liver, duodenum, and cecum were taken at the end of the growing period and rinsed with phosphate buffer saline, then fixed overnight in 10% neutral buffered formalin. The samples were then processed to generate blocks that were then sectioned with rotary microtome (4 μm thick sections), taken on glass slides, and stained with hematoxylin and eosin (HE) [53]. Photomicrographs were taken after examination under light microscope, and certain parameters were measured including (duodenal villi length (DVL), duodenal crypt depth (DCD), cecal mucosal fold length (CMFL), cecal mucosal thickness (CMT), and number of intestinal gland (IG)/ field) [54]. Sections of liver were graded based on the extent of the lesion (0-2= unremarkable, 2–4= mild lesion, 4–6 = moderate lesion, 6 or above= severe lesion) [55].
Statistical Analysis
The data were collected and SPSS statistical software was used for data analysis [56] using a two-way analysis of variance (ANOVA). Breed, group, and breed-group interaction significance were calculated. Differences were considered statistically significant when P ≤ 0.01. The Duncan test [57] and the MSTAT program were used to determine letters for interaction and data were presented as mean ± standard error (SE).
Percentage of Change
To figure out the percentage of change, results of group B (Pb treated group) compared to the starting point group A (the control), while groups C and D (rutin- and RCA NPs-treated groups) results were compared to B group results as original value. Formula by [58, 59] that calculates percentage of change as= ((new value − original value) / original value) ×100 was used.
Results and Discussion
Rutin-Loaded Chitosan Alginate Nanoparticle Characterization
TEM micrographs revealed that RCA NPs were spherical, homogenous, and particle sizes ranged from 20 to 50 nm (Fig. 1). The small particle size of RCA NPs could enhance its physical stability and absorption through the gastrointestinal tract [60]. The DLS measurements of RCA NPs in (Fig. 2) revealed average size of RCA NPs was about 90 ± 4.8 nm with PDI of 0.11 ± 0.02 which is relatively larger than that of TEM measurement since TEM analyzes the sample in its dried state with the original size of sample while DLS is a cumulative analysis of scattered light in aqueous medium [61]. The results also confirmed the narrow size distribution, and homogeneity of the prepared nanoparticles as small PDI value (0.11 ± 0.02) indicates homogeneous dispersion of RCA NPs [62, 63]. The zeta potential value was about +18 mV ± 5.2 which is relatively low positive charge confirming that the negatively charged alginate molecules were successfully incorporated on the surface of the positively charged chitosan through electrostatic attraction in a core-shell structure [64].
Growth Performance
The impact of Pb-induced oxidative stress on the performance of CB and AR breeds varied throughout the six-week experimental period, as shown in Tables 2 and 3. Regarding performance parameters, it was observed that Pb-treated groups exhibited the lowest weekly average BWs, ADG, and FI in both breeds. In comparison to the control groups, final BW values of Pb-treated CB and AR breeds decreased by 36% and 38%, respectively. Additionally, Pb-treated groups showed a substantial decrease in FI accompanied by a significant increase in FCR by 90.98% for CB breed and 94.14% for AR breed when compared to the control groups. Based on these findings, it can be concluded that Pb had a negative impact on broiler growth, including BWG, FI, ADG, and FCR. Similar findings were observed in broiler chicks treated with Pb acetate at dose level 320 mg/kg diet [65], also [66] observed a considerable decrease in BWs of broilers exposed to Pb acetate at a rate of 300 ppm per day compared to the control group. Moreover, parallel findings of lowered growth performance measures were found in broiler chicks treated with Pb acetate at dose level of 400 ppm in drinking water [67]. This decline in performance parameters following Pb exposure may be attributed to alterations in feed consumption and decreased appetites or metabolic disorders, such as inhibition of heme synthesis which leads to cell damage and tissue loss [12], also Pb could induce anxiety, reduction in brain serotonin levels and interruptions of intestinal absorption which all have adverse effect on growth performance [68].
Conversely, rutin- and RCA NPs-treated CB groups demonstrated a significant increase in final BW by 58% and 62%, respectively, when compared to those exposed to Pb. Similarly, both treatments in AR breed showed an increase in the final body weight value by 64% and 68%, respectively. Furthermore, a significant increase of ADG and FI were observed in rutin- and RCA NPs-treated groups during the fourth and fifth weeks of the experimental period in both breeds. Moreover, a notable improvement of FCR were observed in both breeds with reduction of FCR by 48.99% and 50.34% in rutin- and RCA NPs-treated CB, respectively, and by 50.12% and 50.35% in rutin- and RCA NPs-treated AR, respectively. Improvement of performance parameters in groups treated with rutin and RCA NPs may be attributed to the beneficial effects of flavonoids on the gut morphology and its potent antioxidant properties which promote the bird growth [69, 70]. Furthermore, RCA NPs have a more prominent effect on BWG and FCR than rutin alone since alginate chitosan nanoparticles could be a promising system in rutin delivery as small-sized nanoparticles had extraordinary capabilities to get over many anatomical and physiological barriers and deliver rutin locally to sites of interest, thus enhancing growth performance [71, 72]. Despite the enhanced performance of both breeds following treatment with RCA NPs, AR breed achieved superior outcomes starting from the 4th week of treatment compared to CB breed which agreed with [73] who studied the effects of some phytogenic products on broiler growth performance and concluded that the positive impact of these products is primarily observed during the final stage of growth.
Economic Efficiency Measures
Tables 4 and 5 present an economic analysis comparing the effects of rutin and RCA NPs on oxidative stress induced by Pb in both CB and AR broiler breeds. No significant differences related to chick, litter, labor, veterinary management, water, electricity, rent, or miscellaneous costs were found based on TFC results for all groups within both breeds. However, when it comes to feed expenses, rutin- and RCA NPs-treated groups in both breeds had the highest expenditure. This can be explained by the additional cost associated with using rutin and RCA NPs, as highlighted by [74] who found that an increase in feed costs and TC were linked to the added expenses of incorporating phytogenic feed additives in broiler diets. Additionally, [75] revealed that feed costs typically make up around 60-70% of production costs in most farms. Therefore, increasing the feeding expenses ultimately led to increased TVC and TC as overall. Furthermore, rising of feed expenses may be attributed to the increased FI of these groups which is consistent with [76] who reported that adding chitosan powder to broiler diets increases feed intake and thus higher feed costs. Hence, it can be concluded that incorporating rutin and RCA NPs into the diet leads to elevated TC/bird.
Regarding return measures within groups of both breeds, the BW sale of Pb-treated groups had the lowest value which dropped by about 37% in both breeds compared to the control groups due to the lowest final BW in Pb-exposed groups. This is supported by [77] who displayed that only a small portion of ingested Pb is eliminated through the kidneys while most of it stored in the liver and other vital organs which subsequently leads to oxidative damage, impaired function, retarded growth, and economic losses. On the other hand, the BW sales in rutin-treated groups was compensated by 55.27% and 62.37% in CB and AR breeds, respectively, compared to the Pb-treated group while RCA NPs-treated groups revealed a compensation rate of 64.22% and 66.07% in CB and AR breeds, respectively, compared to the Pb-treated group. This highlighting the significance of incorporating rutin and RCA NPs to compensate losses in the BW sales following exposure to Pb. These findings are consistent with [78] who revealed that adding chitosan at 1 g/kg diet mitigated dexamethasone-induced stress via enhancing growth performance, nutrient digestibility, jejunal morphology, and plasma antioxidant enzymes.
In terms of TR, Pb-treated groups in both breeds experienced a significant reduction in TR by approximately 36% compared to the control groups. However, administering rutin resulted in an increase of TR by 54.89% and 61.96% in CB and AR breeds, respectively. While incorporation of RCA NPs into the broiler diet exhibited a notable increase in TR by 63.82% and 65.63% in CB and AR, respectively, when compared to the Pb-treated groups. These findings suggested that Pb-induced oxidative damage could have a negative impact on TR which counteracted through the dietary supplementation of rutin or RCA NPs. This elevation in TR observed in these treated groups of both breeds could be attributed to the significant rise in their BW sales which coincided with [79] who reported that dietary supplementation of 500 and 1000 mg quercetin/kg diet had improved TR in broilers.
Regarding NP findings, there was a significant decrease of NP in Pb-treated groups compared to the control groups whereas CB and AR breeds experienced reductions in NP by 104.48% and 100.76%, respectively. While addition of rutin and RCA NPs to the broilers’ diet resulted in increased NP levels when compared to Pb-treated groups since rutin-treated groups showed an increase of NP by 1594.23% and 10725% in CB and AR breeds, respectively. However, RCA NPs could enhance NP losses following Pb treatment by 1051.92% and 6453.57% in CB and AR breeds, respectively, which attributed to enhanced FCR, as described in our study, leading to increased BW sales, and ultimately improving NP. These findings supported by [80] who proposed that addition of chitosan in geese diet at 200 mg/kg had beneficial effects on nutrient utilization, digestive enzyme activities, FI, FCR, BW sales, and NP.
Concerning economic efficiency measurements such as TR/TC, TC/TR, NP/TC, and NP/TR ratios, the Pb-treated groups displayed the poorest economic efficiency measures as indicated by higher TC/TR ratio which signified that Pb-induced oxidative stress reduced the economic outcomes through higher costs incurred by lower gains while rutin- and RCA NPs-treated groups improved the economic efficiency measures. Additionally, RCA NPs supplementation cost could be reduced on a large scale through using natural resources of chitosan as shrimp shells that subsequently could improve the overall net profit [81]. Conversely, adding rutin alone in broiler diet may reduce the economic outcomes as higher doses, with subsequently higher costs, are required for rutin to effectively exhibit its antioxidant properties [82]. Thus, using RCA nanoparticles to alleviate oxidative stress on a large scale is a more cost-effective option.
When considering the impact of breed, it was observed that CB breed exhibited higher cost parameters such as feed costs, TVC, and TC along with lower TR compared to AR chickens. Furthermore, AR breed outperformed CB in terms of NP and economic efficiency measures. These findings suggested that AR breed has superior features in tolerating Pb toxicity and displaying favorable economic efficiency measures. Our findings align with the study conducted by [83] which exhibited that the AR breed has better performance under various stress factors compared to the CB breed. Similarly, [84] showed that AR breed demonstrated significantly superior performance compared to the Hubbard breed in all stages of production during the hot season in Saudi Arabia. Moreover, [85] reported that AR breed outperformed CB and Lohmann broiler breeds in a comparative study evaluating the BW sales and NP. These findings can probably be linked to growth-associated genes and genetic makeup variations among different strains [86].
Hematological and Biochemical Parameters
Hematological parameters as total erythrocytes count (RBCs) and hemoglobin (Hb) concentration were evaluated in both broiler breeds, as shown in Table 6. According to our findings, Pb-treated groups in both breeds showed a significant decrease in RBCs count and Hb concentration (P<0.01) compared to the control groups whereas Pb-treated groups dropped RBCs count and Hb concentration by 28.5% and 25.7% in CB chickens, respectively, and by 24.3% and 23% in AR chickens, respectively, when compared to the control groups. These findings coincided with [87] who reported that broilers administered 160 mg/kg Pb acetate showed a significant reduction in RBCs count and Hb concentration which may be because of the fact that Pb can markedly shortened the lifespan of circulating RBCs through interfering with several enzymatic steps in the Hb synthetic pathway and increasing RBCs’ membrane fragility [88]. On the other hand, treatment with rutin and RCA NPs notably raised RBCs and Hb concentration (P<0.01) when compared to Pb-treated groups since rutin treatment raised RBCs count and Hb concentration by 14.7% and 13.8%, respectively, in CB breed and by 5.9% and 10% in AR breed compared to the Pb-treated groups. Furthermore, treatment with RCA NPs elevated RBCs count and Hb concentration by 26.2% and 23.7%, respectively, in CB breed and by 23.2% and 18.7% in AR breed as opposed to the Pb-treated groups. These results coincided with [89] who revealed that rutin at dose level of 1 g/kg diet could improve hematological indices in broilers since rutin treatment enhance synthetic pathway of various endogenous proteins including globulins and fibrinogen thus increasing Hb content [90].
Lipid profile including total triglyceride (TG), total cholesterol (TCh), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C) besides total protein (TP) concentration was measured in both broiler breeds (Table 6) and data displayed that Pb-treated groups in both breeds showed a significant increase in TG, TCh, and LDL-C (P<0.01), while HDL-C and TP were markedly decreased (P<0.01) when compared to the control groups. Pb treatment raised TG, TCh, and LDL-C by 34.1%, 25.9%, and 16.9% in CB broilers, respectively, and by 32.2%, 22.5%, and 22.8% in AR broilers, respectively, while reduced HDL-C and TP by 26.1% and 37.2% in CB broilers, respectively, and by 18.9% and 19.7% in AR broilers, respectively, when compared to the control groups. These findings were in the same line with [91] who reported that exposure to Pb acetate at 100 mg/kg BW in broiler chickens resulted in marked elevation of TCh, TG, and LDL-C Triglycerides through activation of cholesterol-biosynthetic enzymes with suppression of cholesterol-catabolic enzymes resulting in hepatic hypercholesterolemia and hypertriglyceridemia, also low level of HDL-C considered one of the most common lipid abnormalities related to heavy metal exposure [92]. Reduction of TP following Pb exposure was consistent with [93] who observed that Pb administered at 284 mg/kg BW in chicken reduced TP significantly which may be due to liver damage caused by Pb exposure, also degradation of synthesized proteins through Pb acetate action on free amino acids [94].
On the other side, rutin and RCA NPs supplementation significantly reduced TG, TCh, and LDL-C (P<0.01), while HDL-C and TP were markedly increased in comparison to Pb-treated groups. Rutin supplementation reduced TG, TCh, and LDL-C by 4.4%, 15.9%, and 7.9% in CB birds, respectively, and by 9.4%, 8.6%, and 7.3% in AR birds, respectively; otherwise, HDL-C and TP were elevated by 10.6% and 23.7% in CB birds, respectively, and by 16.7% and 11.4% in AR birds, respectively, compared to the Pb-treated groups. Correspondingly, RCA NPs supplementation reduced TG, TCh, and LDL-C by 14.2%, 20.6%, and 12.4%, respectively, in CB birds and by 14.4%, 15.7%, and 16.3%, respectively, in AR birds, apart from that HDL-C and TP were elevated by 23.5% and 47.9%, respectively, in CB birds and by 20% and 18.3%, respectively, in AR birds as comparable to the Pb-treated groups. Our data demonstrated that rutin could modulate hypoproteinemia, hypertriglyceridemia, and hypercholesterolemia via improving metabolism and structural integrity through its scavenging activity of free radicals besides it may inhibits cholesterol biosynthetic key enzymes as β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase and decrease the amount of circulating free fatty acids (FFA) available for triacylglycerol synthesis [95, 96]. These findings are consistent with [97] who revealed that rutin at dose level 400 mg/kg diet in broilers had a marked hypolipidemic effect.
Plasma GH concentration was also measured in treated chickens of both breeds as shown in (Table 6) and results displayed that Pb-treated groups in both breeds significantly decreased GH concentration when compared to the control groups whereas GH concentration declined by 26.5% in CB broilers and by 20.7% in AR broilers. This decline could be attributed to prolonged exposure to Pb with subsequent inhibition of GH plasma activity [10]. On the other hand, rutin and RCA NPs supplementation enhanced GH concentration in comparison to the Pb-treated groups, since GH concentration increased by 12.2% and 20.9% in CB and by 7.2% and 13.4% in AR, respectively. Based on these findings, the enhancement of GH is strongly connected to the growth performance of birds, as evidenced by significant improvement in BW and FCR, which may be attributed to the stimulation of GH receptors in skeletal muscle, which in turn increases protein synthesis and stimulates skeletal muscle growth and development, leading to better growth performance [98].
Antioxidant Enzyme Activities and Oxidative Stress Biomarkers
Oxidative damage usually occurs when the antioxidant capability of tissues is overwhelmed by excessive reactive oxygen species (ROS) generation such as superoxide radicals, hydroxyl radicals, and hydrogen peroxides during the metabolic processing of various toxins [99, 100]. The current study investigated the antioxidant defense system in liver tissues of both CB and AR breeds including enzymatic activity of SOD, CAT, and GPx besides non-enzymatic antioxidants as GSH. Additionally, levels of MDA were measured to assess lipid peroxidation, as shown in Table 7. Pb-treated groups of both breeds displayed a significant reduction of GSH, SOD, GPx, and CAT enzymes activity (P<0.01) with a marked increase in MDA level (P<0.01) when compared to the control groups. When assessing each breed separately, Pb-treated CB chickens exhibited reduction of GSH, SOD, GPx, and CAT levels by 48.5%, 59.7%, 50.6%, and 60.7%, respectively, while MDA level was raised by 184.9% when compared to the control group. Correspondingly, Pb-treated AR chickens displayed decline of GSH, SOD, GPx, and CAT levels by 31.8%, 51.2%, 36.9%, and 44.3%, respectively, while MDA level was elevated by 118.9% when compared to the control group. These results coincided with previous study carried by [101] who revealed that Pb could induce liver oxidative damage at dose level of 350 mg/L in drinking water of broiler chickens since Pb inhibits the sulfur containing antioxidant enzymes via making complexes with the sulfhydryl (–SH) groups with subsequent inhibition of their functional activity in scavenging free radicals and peroxides [102].
In contrast, rutin- and RCA NPs-supplemented groups markedly raised GSH, SOD, GPx, and CAT levels (P<0.01) while reduced MDA level (P<0.01) compared to the Pb-treated groups in both breeds. Rutin-treated CB chicken revealed enhanced GSH, SOD, GPx, and CAT levels by 30.4%, 37.5%, 25.2%, and 28.4%, respectively, while MDA level reduced by 21.2% when compared with Pb-treated group. Moreover, RCA NPs-treated CB chicken exhibited elevation of GSH, SOD, GPx, and CAT levels by 66.7%, 72.4%, 61.3%, and 86.3%, respectively, while MDA level declined by 50.7% in comparison to the Pb-treated group. Correspondingly, Rutin-treated AR chicken displayed boosted GSH, SOD, GPx, and CAT levels by 15.5%, 40.9%, 26.2%, and 38.9%, respectively, while MDA level decreased by 24.2% when compared with Pb-treated group. Furthermore, RCA NPs-treated AR chicken unveiled marked rise of GSH, SOD, GPx, and CAT levels by 26.7%, 67.6%, 51.8%, and 69.8%, respectively, while MDA level dropped by 45.7% in comparison to the Pb-treated group. These results come in agreement with previous findings of [103] who revealed that rutin at dose level of 500 mg/kg diet displayed potent antioxidant effects since rutin, as a flavonoid, could inhibit xanthine oxidase activity and lipid peroxidation by scavenging free radicals that attributed to its free hydroxyl groups on A and B rings of rutin carbon skeleton [104]. However, rutin major disadvantage related to its poor bioavailability and stability that may hinder its biological activity so necessitate administration of higher doses to exert its antioxidant properties [70]. Chitosan alginate nanoparticles considered a new natural polymeric vehicle that could improve bioavailability, bio-adhesion, target ability, and sustained release of drugs [21]. Furthermore, these nanoparticles could enhance the antioxidant activity of rutin and maintain its pharmaceutical formulations [105]. In respect to difference between breeds, AR breed showed higher resistance to Pb exposure than CB breed which may attributed to the differential feed efficiency and fat deposition under environmental stressors which in turn could be breed-dependent [38].
Quantitative RT-PCR Analysis
Concerning the relative mRNA expression levels of IGF-I, IGF-II, GHR, and IGFBP genes in the broilers’ liver tissues (Fig. 3), it was demonstrated that Pb treatment reduced their expressions significantly (P < 0.01) by 42.5%, 39.7%, 48.3%, and 48.2%, respectively, in CB breed, and by 33.3%, 33.7%, 45.3%, and 49.5%, respectively, in AR breed compared to control groups. These results are in agreement with [106] who reported that 12.5 μM of Pb acetate could induce marked downregulation of growth-associated genes as IGF-I since exposure to Pb resulted in excessive generation of reactive oxygen species (ROS) which subsequently lead to DNA destruction and alter the relative mRNA expression patterns of theses growth-associated genes [107]. Hence, growth-associated genes including IGF-I and IGF-II play a significant role in poultry growth, body mass conformation, skeletal development, and fat accumulation which mostly expressed in liver and muscle tissues [108]. Therefore, Pb could induce reduction of growth performance in exposed birds.
Meanwhile, rutin- and RCA NPs-treated groups of both breeds mitigated Pb-induced oxidative damage and notably (P < 0.01) enhanced growth-associated genes expression in liver tissue. Rutin upregulated IGF-I, IGF-II, GHR, and IGFBP markedly by 30.96%, 18.24%, 19.92%, and 12.55%, respectively, in CB breed and by 19.64%, 13.88%, 29.25%, and 30.09%, respectively, in AR breed compared to the Pb-treated groups. Correspondingly, RCA NPs were more effective than rutin in reversing oxidative stress induced by Pb as evidenced by significant (P < 0.01) upregulation of growth-associated genes. In the RCA NP-treated groups compared to Pb-treated groups, there was an increase in the gene expression levels of IGF-I, IGF-II, GHR, and IGFBP by 49.04%, 33.99%, 46.62%, and 38.42%, respectively, in CB breed and by 35.98%, 31.67%, 48.63%, and 48.52%, respectively, in AR breed. These findings suggested that rutin quenches the toxicity of Pb acetate though improving the antioxidant mechanisms and serving as a scavenger of free radicals by depressing expression of oxidative stress genes [109], thus decreasing DNA and protein damage that is provoked by ROS generation [110]. Our data also revealed the molecular mechanisms following RCA NPs incorporation into the diet with subsequent enhanced growth performance and economic outcomes as the nutritional status regulates the levels of circulating IGF-I and IGF-II and their expression [111]. Furthermore, GHR plays an important role in improving production traits as it affects GH activity mainly through forming GH-GHR complexes which in turn activate IGF-I secretion in the liver [112]. Moreover, IGFBP plays a crucial role in modulating the action of the IGFs by controlling the half-life of the IGFs in circulation so that a balance is struck between good and bad signals that regulate bird growth rate through development of muscle [113]. Regarding breed effect, AR breed is superior than CB breed as evidenced by significant upregulation of growth-related genes which may be related to the genetic makeup differences among breeds [114].
Histomorphometric Analysis of Intestine and Liver
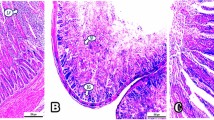
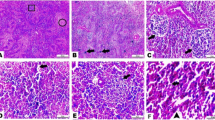
Gut morphology is a crucial parameter for bird health and growth [115] as birds with short intestinal villi or folds have troubles in absorbing essential nutrients [116]. In the present study, examination of intestinal tissue of control groups of CB breed (Fig. 4a) and AR breed (Fig. 4e) showed the duodenal mucosal layer with varying villi lengths (DVL) and crypt depths (DCD). Villi in Pb-treated groups appeared thin with sloughed parts (Fig. 4b and f), but rutin- and RCA NPs-treated groups had a very similar histological structure to the control group (Fig. 4c, d, g, and h). Based on histomorphometric analysis, Pb-treated groups showed the lowest value in DVL and DCD while RCA NPs-treated groups had the tallest villi, and deepest crypts (Fig. 7a and b). Furthermore, the cecal mucosal layers revealed that the mucosal folds were measured along with the tunica mucosa thickness since control groups of both breeds had tall folds with thick mucosa (Fig. 5a and e) while Pb-treated groups had short folds and thin mucosa (Fig. 5b and f). On the other hand, rutin- and RCA NPs-treated groups of both breeds had nearly similar structure to control groups (Fig. 5c, d, g, and h). The cecal mucosal fold length (CMFL) and tunica mucosa thickness (CMT) showed significant differences between groups (P < 0.01) whereas Pb-treated groups showed the shortest folds and the thinnest mucosal layer while RCA NPs-treated groups showed the thickest mucosal layer and the longest folds (Fig. 7c and d). Moreover, number of intestinal glands/fields in both duodenal and cecal tissues was counted in all treated groups; rutin- and RCA NPs-treated groups showed significant increase in number of intestinal glands compared Pb-treated groups since Pb-treated CB breed had the fewest glands (Fig. 7e). These findings revealed that Pb could induce necrosis and desquamation of intestinal villi with subsequent loss of nutrients and weight gain [117]. However, adding rutin to broilers’ diets increased DVL, DVL/DCD ratio, and villi area, resulting in better intestinal function and improved bird growth performance [118]. Moreover, [54] showed that chitosan supplementation at different levels could increase crypt depth and intestinal mucosa thickness, which ensured full-absorption of nutrients.
Liver also plays a key role in metabolism and health of birds so any structural and functional abnormalities in the hepatic tissue could induce negative effects on growth performance [119]. The current study displayed that liver tissue of the control groups showed cords of normal hepatocytes separated by hepatic sinusoids. In contrast, Pb-treated groups showed hepatic degeneration and leukocytic infiltration that moderately resolved in rutin- and RCA NPs-treated groups (Fig. 6 and Fig. 7f). These findings coincided with previous study [120] which revealed that Pb at dose level 50mg/kg feed in CB broiler could interfere with the antioxidant defense mechanism and generating ROS, which mimic degenerative changes and inflammation in the hepatic tissue, while rutin has a good therapeutic effect on liver injury that may be associated with not only its potent antioxidative and anti-inflammatory effects but also through inhibiting lipogenesis and enhancing fatty acid metabolism [95, 121].
Charts showing different measurements, intestinal evaluation of tested groups using DVL (a), DCD (b), CMT (c), and CMFL (d), and IG number/field (e) in addition to liver lesion % (f). Data analyzed through two-way analysis of variance (ANOVA) and different letters indicate statistical significance at (P < 0.01)
Conclusion
Based on the findings of this study, it can be concluded that RCA NPs supplementation at dose level of 50 mg/kg feed could enhance profitability and growth performance within poultry farming operations exposed to environmental stressors. This improvement is mainly attributed to its positive effects on biochemical parameters, antioxidant enzyme activities, gene expression of growth-associated genes and morphological structure of intestinal and liver tissue. Furthermore, breed type could affect growth performance and resistance to various stressors with subsequently economic outcomes since a notable improvement was prominent in AR broiler breed with superior outcomes more than CB breed. Therefore, rutin supplementation especially in the form of RCA NPs is recommended to improve the overall productivity and profitability in poultry farms to encounter challenges associated with oxidative stress, such as Pb toxicity. Moreover, Further studies should be conducted to evaluate the efficiency of RCA NPs supplementation against other immunosuppressive stressors.
Data Availability
Data are available upon request from corresponding author.
References
Khan RU, Durrani F, Chand N, Anwar H (2010) Influence of feed supplementation with Cannabis sativa on quality of broilers carcass. Pakistan Vet J 30:34–38
Talebi A, Asri-Rezaei S, Rozeh-Chai R, Sahraei R (2005) Comparative studies on haematological values of broiler strains (Ross, Cobb, Arbor-acres and Arian). Int J Poult Sci 4:573–579
Verbeke W, Demey V, Bosmans W, Viaene J (2005) Consumer versus producer expectations and motivations related to “superior” quality meat: qualitative research findings. J Food Prod Mark 11:27–41
Estévez M (2015) Oxidative damage to poultry: from farm to fork. Poult Sci 94:1368–1378
Zhang WW, Ma JZ (2011) Waterbirds as bioindicators of wetland heavy metal pollution. Procedia Environ Sci 10:2769–2774
Abou-Kassem D, Mahrose KM, Alagawany M (2016) The role of vitamin E or clay in growing Japanese quail fed diets polluted by cadmium at various levels. Animals 10:508–519
Adonaylo V, Oteiza P (1999) Lead intoxication: antioxidant defenses and oxidative damage in rat brain. Toxicology 135:77–85
Flora SJ, Pande M, Mehta A (2003) Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem Biol Interact 145:267–280
Aendo P, De Garine-Wichatitsky M, Mingkhwan R, Senachai K, Santativongchai P, Krajanglikit P, Tulayakul P (2022) Potential health effects of heavy metals and carcinogenic health risk estimation of pb and cd contaminated eggs from a closed gold mine area in northern Thailand. Foods 11:2791
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Mukherjee A, Kar I, Patra A (2022) Lead toxicity in livestock and poultry: illuminating the molecular pathomechanism, bioaccumulation and effective mitigation strategies. Indian J Anim Health 61:103–118
Abd El-Hack ME, Abdelnour SA, Abd El-Moneim AE-ME, Arif M, Khafaga A, Shaheen H, Samak D, Swelum AA (2019) Putative impacts of phytogenic additives to ameliorate lead toxicity in animal feed. Environ Sci Pollut Res 26:23209–23218
Silva A, Sampaio-Marques B, Fernandes A, Carreto L, Rodrigues F, Holcik M, Santos MA, Ludovico P (2013) Involvement of yeast HSP90 isoforms in response to stress and cell death induced by acetic acid. PloS One 8:e71294
Mohammadi Gheisar M, Kim IH (2018) Phytobiotics in poultry and swine nutrition–a review. Ital J Anim Sci 17:92–99
Giannenas I, Bonos E, Christaki E, Florou-Paneri P (2013) Essential oils and their applications in animal nutrition. Med Aromat Plants 2:140
Mosoni L, Balage M, Vazeille E, Combaret L, Morand C, Zagol-Ikapitte I, Boutaud O, Marzani B, Papet I, Dardevet D (2010) Antioxidant supplementation had positive effects in old rat muscle, but through better oxidative status in other organs. Nutrition 26:1157–1162
Long S, He T, Wu D, Yang M, Piao X (2020) Forsythia suspensa extract enhances performance via the improvement of nutrient digestibility, antioxidant status, anti-inflammatory function, and gut morphology in broilers. Poult Sci 99:4217–4226
Yang S, Zhang J, Jiang Y, Xu Y, Jin X, Yan S, Shi B, Shini S (2021) Effects of dietary supplementation with Artemisia argyi alcohol extract on growth performance, blood biochemical properties and small intestinal immune markers of broilers challenged with lipopolysaccharide. Anim Prod Sci 62:234–247
Gullón P, Bilal U, Cebrecos A, Badland HM, Galán I, Franco M (2017) Intersection of neighborhood dynamics and socioeconomic status in small-area walkability: the Heart Healthy Hoods project. Int J Health Geogr 16:1–9
Khan H, Ullah H, Martorell M, Valdes S, Belwal T, Tejada S, Sureda A, Kamal MA (2021) Flavonoids nanoparticles in cancer: treatment, prevention and clinical prospects. Semin Cancer Biol 69:200–211
Rahaiee S, Hashemi M, Shojaosadati SA, Moini S, Razavi SH (2017) Nanoparticles based on crocin loaded chitosan-alginate biopolymers: antioxidant activities, bioavailability and anticancer properties. Int J Biol Macromol 99:401–408
Khambualai O, Yamauchi K, Tangtaweewipat S, Cheva-Isarakul B (2009) Growth performance and intestinal histology in broiler chickens fed with dietary chitosan. Br Poultry Sci 50:592–597
Ge H, Hua T, Chen X (2016) Selective adsorption of lead on grafted and crosslinked chitosan nanoparticles prepared by using Pb2+ as template. J Hazard Mater 308:225–232
Aluani D, Tzankova V, Kondeva-Burdina M, Yordanov Y, Nikolova E, Odzhakov F, Apostolov A, Markova T, Yoncheva K (2017) Еvaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int J Biol Macromol 103:771–782
Nalini T, Basha SK, Sadiq AMM, Kumari VS, Kaviyarasu K (2019) Development and characterization of alginate/chitosan nanoparticulate system for hydrophobic drug encapsulation. J Drug Deliv Sci Technol 52:65–72
Yacamán MJ, Ascencio J, Liu H, Gardea-Torresdey J (2001) Structure shape and stability of nanometric sized particles. J Vac Sci Technol B: Microelectronics Nanometer Structures Processing, Measurement, Phenomena 19:1091–1103
Dale N (1994) National research council nutrient requirements of poultry–ninth revised edition (1994). J Appl Poultry Res 3:101
Jiao W-B, Accinelli GG, Hartwig B, Kiefer C, Baker D, Severing E, Willing E-M, Piednoel M, Woetzel S, Madrid-Herrero E (2017) Improving and correcting the contiguity of long-read genome assemblies of three plant species using optical mapping and chromosome conformation capture data. Genome Res 27:778–786
Hassan FA, Roushdy EM, Kishawy AT, Zaglool AW, Tukur HA, Saadeldin IM (2018) Growth performance, antioxidant capacity, lipid-related transcript expression and the economics of broiler chickens fed different levels of rutin. Animals. 9:7
Kandemir FM, Caglayan C, Aksu EH, Yildirim S, Kucukler S, Gur C, Eser G (2020) Protective effect of rutin on mercuric chloride-induced reproductive damage in male rats. Andrologia 52:e13524
Abang F, Aker D, Odunlade T (2016) Economic of production of growing Japanese quails (Coturnix coturnix japonica) fed sun-dried mango (Mangifera indica) kernel meal. Global J Agric Res 4:48–55
Omar MAE (2009) Economic study on the productive and reproductive efficiency in dairy farms in relation to veterinary management. Ph. D. of Vet. Medical Science, Zagazig University, Egypt
Liza S (2012) Effect of days open, dry period and calving interval on economic and productive efficiency of dairy farms. MV Sc, Faculty of Veterinary Medicine Banha University–Egypt
Ani A, Ugwuowo L (2011) Response of weaner rabbits to diets containing graded levels of processed velvet beans (Mucuna pruriens). Afr J Biotechnol 10:14984–14989
Amarapurkar S, Murthy C, Naik B (2014) Costs and returns in commercial broiler rearing in Dharwad district. Int J Commer Bus Manag 7:316–319
Omar MA (2014) Economic evaluation of probiotic (Lactobacillus acidophilus) using in different broiler breeds within Egypt. Benha Vet Med J 26:52–60
Kamel E, Abdel-Fattah F, El-Qaliouby H, Mahmoud E (2016) Response of New Zealand rabbits to diet containing guava waste (Psidium guaijava L.): 1. effect on growth performance, diet digestibility and economic efficiency. Alex J Vet Sci 50:24–35
Sallam EA, Mohammed LS, Elbasuni SS, Azam AE, Soliman MM, Science (2021) Impacts of microbial based therapy on growth performance, intestinal health, carcass traits and economic efficiency of clostridium perfringens-infected cobb and arbor acres broilers. Vet Med 7: 773–791
El-Sheikh S, Al-Shokiry N, Salama A, Khidr R (2013) Utilization of Azzawi date meal in local laying hen diets. Egypt Poult Sci J 33:1115–1127
Atallah S (2004) Effect of cattle diseases on reproductive, productive and economic efficiency of dairy farms. Minufiya Vet J 1:99–114
Kamel ER, Mohammed LS, Abdelfattah FA, production (2020) Effect of a diet containing date pits on growth performance, diet digestibility, and economic evaluation of Japanese quail (Coturnix coturnix japonica). Trop Anim Health 52:339–346
Marcu A, Vacaru-Opriş I, Dumitrescu G, Ciochină LP, Marcu A, Nicula M, Peţ I, Dronca D, Kelciov B, Mariş C (2013) The influence of genetics on economic efficiency of broiler chickens growth. Anim Sci Biotechnol 46:339–346
Regassa SL, Bekana E, Geleta T (2013) Production performances of a fayoumi chicken breed under backyard management condition in Mid Rift Valley of Ethiopia. Herald J Agric Food Sci Res 2:78–81
Alagawany M, Abd El-Hack M, Farag M, Shaheen H, Abdel-Latif M, Noreldin A, Patra A (2018) The usefulness of oregano and its derivatives in poultry nutrition. Worlds Poult Sci J 74:463–474
Feldman HM, Dollaghan CA, Campbell TF, Kurs-Lasky M, Janosky JE, Paradise JL (2000) Measurement properties of the MacArthur Communicative Development Inventories at ages one and two years. Child Dev 71:310–322
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22:266–282
Odukoya O, Sofidiya M, Ilori O, Gbededo M, Ajadotuigwe J, Olaleye O, Bouskela E, Cyrino F, Marcelon G, Brinkhaus B (1994) Malondialdehyde determination as index of lipid peroxidation. Int J Biol Chem 3:281–285
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25:192–205
Aebi H, Mörikofer-Zwez S, von Wartburg J-P (1972) Alternative molecular forms of erythrocyte catalase. In: Structure and Function of Oxidation–Reduction Enzymes. Elsevier, pp 345–351
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pfaffl M (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Anderson G, Bancroft J (2002) Tissue processing and microtomy. Theory and Practice of histological techniques 5:85–99
Ayman U, Akter L, Islam R, Bhakta S, Rahman MA, Islam MR, Sultana N, Sharif A, Jahan MR, Rahman MS (2022) Dietary chitosan oligosaccharides improves health status in broilers for safe poultry meat production. Ann Agric Sci 67:90–98
Gowda N, Ledoux D, Rottinghaus G, Bermudez A, Chen Y (2008) Efficacy of turmeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult Sci 87:1125–1130
IBM Corp N (2017) IBM SPSS statistics for windows, Version 25.0. IBM corp, Armonk, NY
Duncan OD, Duncan B (1955) A methodological analysis of segregation indexes. Am Sociol Rev 20:210–217
Vartia YO (1976) Relative changes and index numbers. ETLA A
Vickers AJ (2001) The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol 1:1–4
Varshosaz J, Taymouri S, Jahanian-Najafabadi A, Alizadeh A (2018) Efavirenz oral delivery via lipid nanocapsules: formulation, optimisation, and ex-vivo gut permeation study. IET Nanobiotechnol 12:795–806
Ghazy O, Fouad M, Saleh H, Kholif A, Morsy T (2021) Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem 341:128259
Hebeish A, El-Rafie M, El-Sheikh MA, El-Naggar ME (2014) Ultra-fine characteristics of starch nanoparticles prepared using native starch with and without surfactant. J Inorg Organomet Polym Mater Lett 24:515–524
Ameen F, Abdullah MM, Al-Homaidan AA, Al-Lohedan HA, Al-Ghanayem AA, Almansob A (2020) Fabrication of silver nanoparticles employing the cyanobacterium Spirulina platensis and its bactericidal effect against opportunistic nosocomial pathogens of the respiratory tract. J Mol Struct 1217:128392
Liu Q, Qin Y, Jiang B, Chen J, Zhang T (2022) Development of self-assembled zein-fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surf B Biointerfaces 216:112529
Abdullah S, Mustafa N (2009) Effects of lead acetate and probiotic on some physiological parameters in broiler chicks. Rafidain J Sci 20:1–7
Pavani V, Lakshman M, Madhuri D, Gopalareddy A (2022) Effect of lead and thiram toxicity on body weights of broilers. J Pharm Innov 11:1172–1174
Karimi I, Nasr J, Zanganeh F (2013) Protective effects of an alfalfa aqueous extract on lead toxicity in broiler chickens: a biochemical approach. Comp Clin Pathol 22:1129–1136
Haider S, Saleem S, Tabassum S, Khaliq S, Shamim S, Batool Z, Parveen T, Q-u-a I, Haleem DJ (2013) Alteration in plasma corticosterone levels following long term oral administration of lead produces depression like symptoms in rats. Metab Brain Dis 28:85–92
Viveros A, Chamorro S, Pizarro M, Arija I, Centeno C, Brenes A (2011) Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci 90:566–578
Gullon B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G (2017) Rutin: a review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Technol 67:220–235
Meimandipour A, Nouri Emamzadeh A, Soleimani A (2017) Effects of nanoencapsulated aloe vera, dill and nettle root extract as feed antibiotic substitutes in broiler chickens. Arch Anim Breed 60:1–7. https://doi.org/10.5194/aab-60-1-2017
Abou El-Maaty A, Hayam M, El-Aziz A, El-Diasty M, El-Said E (2021) Effect of dietary rocket seeds meal and nano-chitosan on performance and related gene expression to growth and lipid profile in broiler chicks. Egypt J Nutr Feeds 24:139–155
Mountzouris K, Paraskevas V, Tsirtsikos P, Palamidi I, Steiner T, Schatzmayr G, Fegeros K (2011) Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim Feed Sci Technol 168:223–231
Shahin S, Ibrahim D, Badawi M (2020) Effects of phytogenic supplementation on productive and economic performance in broilers. J Anim Health Prod 9:42–49
Thirumalaisamy G, Muralidharan J, Senthilkumar S, Hema Sayee R, Priyadharsini M (2016) Cost-effective feeding of poultry. Int J Sci Environ Technol 5:3997–4005
Khattak F, Ronchi A, Castelli P, Sparks N (2014) Effects of natural blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult Sci 93:132–137
Rahman MM, Mahmud H, Ali MR, Rahman SZ, Mollah MAB, Costa SG (2021) Assessment of heavy metal soil pollution in the agricultural land of North Western Bangladesh. Mod Cartogr Ser 10:221–242
Osho S, Adeola O (2020) Chitosan oligosaccharide supplementation alleviates stress stimulated by in-feed dexamethasone in broiler chickens. Poult Sci 99:2061–2067
Zhang S, Kim IH (2020) Effect of quercetin (flavonoid) supplementation on growth performance, meat stability, and immunological response in broiler chickens. Livest Sci 242:104286
Miao Z, Liu Y, Guo L, Zhao W, Zhang J (2020) Effects of dietary chitosan on growth rate, small intestinal morphology, nutrients apparent utilization and digestive enzyme activities of growing Huoyan geese. Animal 14:2635–2641
Abomosallam M, Elalfy M, Zheng Z, Nagata K, Suzuki M (2022) Adsorption kinetics and thermodynamics of toxic metal ions onto chitosan nanoparticles extracted from shrimp shells. Nanotechnol Environ Eng 7:1–13
Chen D, Liu Y, Liu P, Zhou Y, Jiang L, Yuan C, Huang M (2022) Orally delivered rutin in lipid-based nano-formulation exerts strong antithrombotic effects by protein disulfide isomerase inhibition. Drug Deliv 29:1824–1835
Saki A, Momeni M, Tabatabaei M, Ahmadi A, Rahmati M, Matin HH, Janjan A (2010) Effect of feeding programs on broilers Cobb and Arbor Acres plus performance. Int J Poult Sci 9:795–800
Attia YA, Al-Tahawy WS, de Oliveira MC, Al-Harthi MA, El-Din AAET, Hassan MI (2015) Response of two broiler strains to four feeding regimens under hot climate. Anim Prod Sci 56:1475–1483
Das S, Islam MA (2023) Comparative growth, meat yield and blood lipid profiles of arbor acres, cobb 500 and lohmann broiler strains International Journal of Agriculture. Environ Biores 8:55–63
Obike K, Amusa T, Olowolafe H (2017) Risk management and determinants of farm output among small scale poultry farmers in Ekiti State, Nigeria. Agro-Sci 16:9–16
Cai P, Zhu Q, Cao Q, Bai Y, Zou H, Gu J, Yuan Y, Liu X, Liu Z, Bian J (2021) Quercetin and allicin can alleviate the hepatotoxicity of lead (Pb) through the PI3K signaling pathway. J Agric Food Chem 69:9451–9460
Mohanty JG, Nagababu E, Rifkind JM (2014) Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol 5:84
Awad A, Zaglool AW, Khalil SR (2018) Immunohaematological status and mRNA expression of the genes encoding interleukin-6, nuclear-factor kappa B, and tumor-necrosis factor-α in the spleen of broilers supplemented with dietary rutin. Anim Prod Sci 59:1454–1461
Rakshit S, Shukla P, Verma A, Kumar Nirala S, Bhadauria M (2021) Protective role of rutin against combined exposure to lipopolysaccharide and D-galactosamine-induced dysfunctions in liver, kidney, and brain: Hematological, biochemical, and histological evidences. J Food Biochem 45:e13605
Hossain MA, Akanda MR, Mostofa M, Awal MA (2014) Therapeutic competence of dried garlic powder (Allium sativum) on biochemical parameters in lead (Pb) exposed broiler chickens. J Adv Vet Anim Res 1:189–195
Hashem MA, Abd El Hamied SS, Ahmed EM, Amer SA, El-Sharnouby ME (2021) Mitigating the growth, biochemical changes, genotoxic and pathological effects of copper toxicity in broiler chickens by supplementing vitamins C and E. Animals. 11:1811
Hussain S, Ali S, Mumtaz S, Shakir HA, Ahmad F, Tahir HM, Ulhaq M, Khan MA, Zahid MT (2020) Dose and duration-dependent toxicological evaluation of lead acetate in chicks. Environ Sci Pollut Res 27:15149–15164
Highab S, Aliyu M, Muhammad B (2018) Effect of resveratrol on liver histopathology of lead-induced toxicity in wistar rats. J Pharm Res Int 20:1–8
Liu Q, Pan R, Ding L, Zhang F, Hu L, Ding B, Zhu L, Xia Y, Dou X (2017) Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int Immunopharmacol 49:132–141
Bazyar H, Moradi L, Zaman F, Zare Javid A (2023) The effects of rutin flavonoid supplement on glycemic status, lipid profile, atherogenic index of plasma, brain-derived neurotrophic factor (BDNF), some serum inflammatory, and oxidative stress factors in patients with type 2 diabetes mellitus: A double-blind, placebo-controlled trial. Phytother Res 37:271–284
Li Y, Mei H, Liu Y, Li Z, Qamar H, Yu M, Ma X (2023) Dietary supplementation with rutin alters meat quality, fatty acid profile, antioxidant capacity, and expression levels of genes associated with lipid metabolism in breast muscle of Qingyuan partridge chickens. Foods. 12:2302
Chen P, Li S, Zhou Z, Wang X, Shi D, Li Z, Li X, Xiao Y (2022) Liver fat metabolism of broilers regulated by Bacillus amyloliquefaciens TL via stimulating IGF-1 secretion and regulating the IGF signaling pathway. Front Microbiol 13:958112
Sirajudeen M, Gopi K, Tyagi JS, Moudgal RP, Mohan J, Singh R (2011) Protective effects of melatonin in reduction of oxidative damage and immunosuppression induced by aflatoxin B1-contaminated diets in young chicks. Environ Toxicol 26:153–160
Xiong Y, Chu X, Yu T, Knoedler S, Schroeter A, Lu L, Zha K, Lin Z, Jiang D, Rinkevich Y (2023) Reactive oxygen species-scavenging nanosystems in the treatment of diabetic wounds. Adv Healthc Mater 12:2300779
Seven İ, Aksu T, Seven PT (2012) The effects of propolis and vitamin C supplemented feed on performance, nutrient utilization and carcass characteristics in broilers exposed to lead. Livest Sci 148:10–15
Mutlu SI, Seven I, Birben N, Arslan A, Seven PT (2021) Alleviation potential of quercetin in laying quails exposed to lead: effects on productive performance, egg quality, cecal microflora, and nutrient digestibility. J Environ Agric Sci 10:20–27
Li H, Gu Y, Jin R, He Q, Zhou Y (2022) Effects of dietary rutin supplementation on the intestinal morphology, antioxidant capacity, immunity, and microbiota of aged laying hens. Antioxidants 11:1843
Kirschweng B, Tilinger DM, Hégely B, Samu G, Tátraaljai D, Földes E, Pukánszky B (2018) Melt stabilization of PE with natural antioxidants: comparison of rutin and quercetin. Eur Polym J 103:228–237
Surendran V, Palei NN (2022) Formulation and characterization of rutin loaded chitosan-alginate nanoparticles: antidiabetic and cytotoxicity studies. Curr Drug Deliv 19:379–394
Yin K, Cui Y, Sun T, Qi X, Zhang Y, Lin H (2020) Antagonistic effect of selenium on lead-induced neutrophil apoptosis in chickens via miR-16-5p targeting of PiK3R1 and IGF1R. Chemosphere 246:125794
Ebrahimi R, Faseleh Jahromi M, Liang JB, Soleimani Farjam A, Shokryazdan P, Idrus Z (2015) Effect of dietary lead on intestinal nutrient transporters mRNA expression in broiler chickens. Biomed Res Int 3:240
El-Saway HB, Soliman MM, Sadek KM, Nassef E, Abouzed TK (2022) Beneficial impact of dietary methyl methionine sulfonium chloride and/or L-carnitine supplementation on growth performance, feed efficiency, and serum biochemical parameters in broiler chicken: role of IGF-1 and MSTN genes. Tropl Anim Health Prod 54:98
Al-Rejaie SS, Aleisa AM, Sayed-Ahmed MM, Al-Shabanah OA, Abuohashish HM, Ahmed MM, Al-Hosaini KA, Hafez MM (2013) Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement Altern Med 13:1–9
Dogra A, Kour D, Gour A, Bhardwaj M, Bag S, Dhiman SK, Kumar A, Singh G, Nandi U (2022) Ameliorating effect of rutin against diclofenac-induced cardiac injury in rats with underlying function of FABP3, MYL3, and ANP. Drug Chem Toxicol 46:597–608
Yu J, Yang H, Wang Z, Dai H, Xu L, Ling C (2018) Effects of arginine on the growth performance, hormones, digestive organ development and intestinal morphology in the early growth stage of layer chickens. Ital J Anim Sci 17:1077–1082
Zhang L, Lin S, An L, Ma J, Qiu F, Jia R, Nie Q, Zhang D, Luo Q, Li T (2016) Chicken GHR natural antisense transcript regulates GHR mRNA in LMH cells. Oncotarget 7:73607
Kim JW (2010) The endocrine regulation of chicken growth. Asian Australas J Anim Sci 23:1668–1676
Larkina TA, Barkova OY, Peglivanyan GK, Mitrofanova OV, Dementieva NV, Stanishevskaya OI, Vakhrameev AB, Makarova AV, Shcherbakov YS, Pozovnikova MV (2021) Evolutionary subdivision of domestic chickens: implications for local breeds as assessed by phenotype and genotype in comparison to commercial and fancy breeds. Agriculture 11:914
Xu Z, Hu C, Xia M, Zhan X, Wang M (2003) Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci 82:1030–1036
Li J, Cheng Y, Chen Y, Qu H, Zhao Y, Wen C, Zhou Y (2019) Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals 9:493
Sinan KI, Akpulat U, Aldahish AA, Celik Altunoglu Y, Baloğlu MC, Zheleva-Dimitrova D, Gevrenova R, Lobine D, Mahomoodally MF, Etienne OK (2021) LC-MS/HRMS analysis, anti-cancer, anti-enzymatic and anti-oxidant effects of boerhavia diffusa extracts: a potential raw material for functional applications. Antioxidants 10:2003
Chen S, Liu H, Zhang J, Zhou B, He X, Wang T, Wang C (2023) Dietary rutin improves breast meat quality in heat-stressed broilers and protects mitochondria from oxidative attack via the AMPK/PINK1-Parkin pathway. J Sci Food Agric 103:2367–2377
Niu J, Wang Q, Jing C, Liu Y, Liu H, Jiao N, Huang L, Jiang S, Guan Q, Li Y (2022) Dietary Galla chinensis tannic acid supplementation in the diets improves growth performance, immune function and liver health status of broiler chicken. Front Vet Sci 9:1024430
Monjur T, Rahaman T, Islam M, Ferdous K (2018) Effects of dietary supplementation of lead (Pb) on biochemical, gross and histo-morphological changes in different organs of broiler chicken. Bangladesh J Vet Med 16:137–144
Liang W, Zhang D, Kang J, Meng X, Yang J, Yang L, Xue N, Gao Q, Han S, Gou X (2018) Protective effects of rutin on liver injury in type 2 diabetic db/db mice. Biomed Pharmacother 107:721–728
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
N. W. analyzed the economic efficiency measures, designed this study and wrote the manuscript, M. A. analyzed oxidative stress biomarkers, organized the concept of this study and wrote the manuscript. B. H. performed qRT-PCR analysis of growth associated genes. Z. S. performed histomorphometric and histopathological analysis. N. H. analyzed biochemical and hematological parameters. S. S. performed growth performance analysis. All authors also read, revise and approved the article for publication.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the animals’ ethical approval committee of Mansoura University, (MU-ACUC), No. (VM.R.22.11.28).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wahed, N.M., Abomosallam, M., Hendam, B.M. et al. Economic and Productive Comparison of Rutin and Rutin-Loaded Chitosan Alginate Nanoparticles Against Lead-Induced Oxidative Stress in Cobb and Arbor Broiler Breeds. Biol Trace Elem Res 202, 4715–4734 (2024). https://doi.org/10.1007/s12011-023-04019-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-04019-x