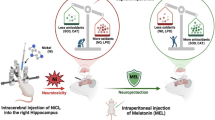
Abstract
Arsenic (As) exposure is known to cause several neurological disorders through various molecular mechanisms such as oxidative stress, apoptosis, and autophagy. In the current study, we assessed the effect of melatonin (Mel) on As-induced neurotoxicity. Thirty male Wistar rat were treated daily for 28 consecutive days. As (15 mg/kg, gavage) and Mel (10 and 20 mg/kg, i.p.) were administered to rats. Morris water maze test was done to evaluate learning and memory impairment in training days and probe trial. Oxidative stress markers including MDA and GSH levels, SOD activity, and HO-1 levels were measured. Besides, the levels of apoptosis (caspase 3, Bax/Bcl2 ratio) and autophagy markers (Sirt1, Beclin-1, and LC3 II/I ratio) as well as the expression of miR-144 and miR-34a in cortex tissue were determined. As exposure disturbed learning and memory in animals and Mel alleviated these effects. Also, Mel recovered cortex pathological damages and oxidative stress induced by As. Furthermore, As increased the levels of apoptosis and autophagy proteins in cortex, while Mel (20 mg/kg) decreased apoptosis and autophagy. Also, Mel increased the expression of miR-144 and miR-34a which inhibited by As. In conclusion, Mel administration attenuated As-induced neurotoxicity through anti-oxidative, anti-apoptotic, and anti-autophagy mechanisms, which may be recommended as a therapeutic target for neurological disorders.









Similar content being viewed by others
Change history
03 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12011-023-04051-x
Abbreviations
- AChE:
-
Acetyl choline esterase
- Bax:
-
Bcl-2-associated X protein
- BBB:
-
Blood brain barrier
- Bcl-2:
-
B-cell lymphoma 2
- BSA:
-
Bovine serum albumin
- cDNA:
-
Complementary DNA
- CT:
-
Cycle threshold
- DTNB 5:
-
5′-dithiobis 2-nitrobenzoic acid
- EDTA:
-
Ethylene diamine tetraacetic acid
- EGTA:
-
Ethylene glycol tetraacetic acid
- GSH:
-
Glutathione
- H&E:
-
Hematoxylin and eosin
- HO-1:
-
Heme oxygenase-1
- LC3:
-
Microtubule-associated protein 1 light chain 3
- LPS:
-
Lipopolysaccharide
- MDA:
-
Malondialdehyde
- Mel:
-
Melatonin
- ml:
-
Milliliter
- miR:
-
MicroRNA
- mTOR:
-
Mechanistic target of rapamycin
- MWM:
-
Morris water maze
- Nrf2:
-
Nuclear factor-carotenoid 2 related factor 2
- PBS:
-
Phosphate buffer saline
- PIC:
-
Phosphatase inhibitor cocktail
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PVDF:
-
Polyvinylidene fluoride
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- SDS:
-
Sodium dodecyl sulfate
- Sirt1:
-
Sirtuin
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TBST:
-
Tris buffered saline tween
- TMED:
-
Tetramethylethylenediamine
References
Najafi N, Rezaee R, Hayes AW, Karimi G (2022) A review of mechanisms underlying the protective effects of natural compounds against arsenic-induced neurotoxicity. BioMetals 36(4):799–813. https://doi.org/10.1007/s10534-022-00482-6
Jomova K, Jenisova Z, Feszterova M et al (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107. https://doi.org/10.1002/jat.1649
Garza-Lombó C, Pappa A, Panayiotidis MI et al (2019) Arsenic-induced neurotoxicity: a mechanistic appraisal. J Biol Inorg Chem 24:1305–1316. https://doi.org/10.1007/s00775-019-01740-8
Ran S, Liu J, Li S (2020) A systematic review of the various effect of arsenic on glutathione synthesis in vitro and in vivo. Biomed Res Int 2020:9414196. https://doi.org/10.1155/2020/9414196
Hu Y, Li J, Lou B et al (2020) The role of reactive oxygen species in arsenic toxicity. Biomolecules 10. https://doi.org/10.3390/biom10020240
Kirilovsky ER, Anguiano OL, Bongiovanni GA, Ferrari A (2022) Effects of acute arsenic exposure in two different populations of Hyalella curvispina amphipods from North Patagonia Argentina. J Toxicol Environ Health 85:71–88
Thakur M, Rachamalla M, Niyogi S et al (2021) Molecular mechanism of arsenic-induced neurotoxicity including neuronal dysfunctions. Int J Mol Sci:22. https://doi.org/10.3390/ijms221810077
Arora MK, Singh D, Tomar R, Jangra A (2022) Neuroprotective efficacy of edaravone against arsenic-induced behavioral and neurochemical deficits in rats: amelioration of cholinergic and mitochondrial functions. CNS Neurol Disord Drug Targets. https://doi.org/10.2174/1871527321666220225112241
Li B, Xia M, Zorec R et al (2021) Astrocytes in heavy metal neurotoxicity and neurodegeneration. Brain Res 1752:147234. https://doi.org/10.1016/j.brainres.2020.147234
O’Callaghan JP, Sriram K (2005) Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf 4:433–442
Singh Sankhla M (2018) Arsenic-induced neurotoxic & carcinogenic effects on humans. Open Acc J of Toxicol 3:4–7. https://doi.org/10.19080/oajt.2018.03.555617
Bali İ, Bilir B, Emir S, Turan F, Yılmaz A, Gökkuş T, Aydın M (2016) The effects of melatonin on liver functions in arsenic-induced liver damage. Turkish Journal of Surgery/Ulus Cerrahi Derg 32(4):233. https://doi.org/10.5152/UCD.2015.3224
Kadeyala PK, Sannadi S, Gottipolu RR (2013) Alterations in apoptotic caspases and antioxidant enzymes in arsenic exposed rat brain regions: reversal effect of essential metals and a chelating agent. Environ Toxicol Pharmacol 36:1150–1166. https://doi.org/10.1016/j.etap.2013.09.021
Mozaffarian F, Dehghani MA, Vanani AR, Mahdavinia M (2021) Protective effects of alpha lipoic acid against arsenic induced oxidative stress in isolated rat liver mitochondria. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02712-3
Zhang Y, Wei Z, Liu W et al (2017) Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget 8:3773
Wu S, Rao G, Wang R et al (2021) The neuroprotective effect of curcumin against ATO triggered neurotoxicity through Nrf2 and NF-κB signaling pathway in the brain of ducks. Ecotoxicol Environ Saf 228:112965. https://doi.org/10.1016/j.ecoenv.2021.112965
Abdollahzade N, Babri S, Majidinia M (2021) Attenuation of chronic arsenic neurotoxicity via melatonin in male offspring of maternal rats exposed to arsenic during conception: involvement of oxidative DNA damage and inflammatory signaling cascades. Life Sci 266:118876. https://doi.org/10.1016/j.lfs.2020.118876
Najafi N, Ghasemzadeh Rahbardar M, Hosseinzadeh H et al (2022) Chemical agents protective against rotenone-induced neurotoxicity. Toxicol Environ Chem 104:149–175
Pastorek M, Gronesova P, Cholujova D et al (2014) Realgar (As4S4) nanoparticles and arsenic trioxide (As2O3) induced autophagy and apoptosis in human melanoma cells in vitro. Neoplasma 61:700–709. https://doi.org/10.4149/neo_2014_085
Herbert KJ, Holloway A, Cook AL et al (2014) Arsenic exposure disrupts epigenetic regulation of SIRT1 in human keratinocytes. Toxicol Appl Pharmacol 281:136–145. https://doi.org/10.1016/j.taap.2014.09.012
Shen X, Zhi F, Shi C et al (2023) The involvement and therapeutic potential of lncRNA Kcnq1ot1/miR-34a-5p/Sirt1 pathway in arsenic trioxide-induced cardiotoxicity. J Transl Med 21:1–15
Yarmohammadi F, Barangi S, Aghaee-Bakhtiari SH, Hosseinzadeh H, Moosavi Z, Reiter RJ, Hayes AW, Mehri S, Karimi G (2023) Melatonin ameliorates arsenic-induced cardiotoxicity through the regulation of the Sirt1/Nrf2 pathway in rats. BioFactors 49(3):620–635. https://doi.org/10.1002/biof.1934
Hao R, Ge J, Song X et al (2022) Cadmium induces ferroptosis and apoptosis by modulating miR-34a-5p/Sirt1axis in PC12 cells. Environ Toxicol 37:41–51
Chen P, Chen F, Lei J et al (2019) Activation of the miR-34a-mediated SIRT1/mTOR signaling pathway by urolithin A attenuates D-galactose-induced brain aging in mice. Neurotherapeutics 16:1269–1282
Azzimato V, Chen P, Barreby E et al (2021) Hepatic miR-144 drives fumarase activity preventing NRF2 activation during obesity. Gastroenterology 161:1982–1997
Zhang S, Liu X, Wang J et al (2022) Targeting ferroptosis with miR-144-3p to attenuate pancreatic β cells dysfunction via regulating USP22/SIRT1 in type 2 diabetes. Diabetol Metab Syndr 14:1–14
Chen N, Chu S, Zhou X et al (2019) Ginsenoside Rg1 protects against ischemic/reperfusion-induced neurotoxicity through miR-144/Nrf2/ARE pathway. FASEB J 33:500–514
Karami A, Darreh-Shori T, Schultzberg M, Eriksdotter M (2021) CSF and plasma cholinergic markers in patients with cognitive impairment. Front Aging Neurosci 13:704583
Yadav RS, Chandravanshi LP, Shukla RK et al (2011) Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicology 32:760–768. https://doi.org/10.1016/j.neuro.2011.07.004
Korkmaz A, Reiter RJ, Topal T et al (2009) Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med 15:43–50
Jiang Y, Shi H, Liu Y, Zhao S, Zhao H (2021) Applications of melatonin in female reproduction in the context of oxidative stress. Oxid med cell longev 2021:6668365. https://doi.org/10.1155/2021/6668365
Erdoğan MM, Erdemli ME, Özhan O et al (2023) Effect of melatonin on increasing the effectiveness of liver preservation solution. Turk J Gastroenterol 34:943–951
Brzozowski T, Konturek PC, Zwirska-Korczala K et al (2005) Importance of the pineal gland, endogenous prostaglandins and sensory nerves in the gastroprotective actions of central and peripheral melatonin against stress-induced damage. J Pineal Res 39:375–385
Roy J, Wong KY, Aquili L et al (2022) Role of melatonin in Alzheimer’s disease: from preclinical studies to novel melatonin-based therapies. Front Neuroendocrinol 65:100986
Deng Y, Jiao C, Mi C et al (2015) Melatonin inhibits manganese-induced motor dysfunction and neuronal loss in mice: involvement of oxidative stress and dopaminergic neurodegeneration. Mol Neurobiol 51:68–88. https://doi.org/10.1007/s12035-014-8789-3
Shen X, Tang C, Wei C et al (2022) Melatonin induces autophagy in amyotrophic lateral sclerosis mice via upregulation of SIRT1. Mol Neurobiol 59:4747–4760
Escribano BM, Colin-Gonzalez AL, Santamaría A, Túnez I (2014) The role of melatonin in multiple sclerosis, Huntington’s disease and cerebral ischemia. CNS Neurol Disord Drug Targets 13(6):1096–1119. https://doi.org/10.2174/1871527313666140806160400
Abdollahzade N, Mihanfar A, Majidinia M (2022) Molecular mechanisms underlying ameliorative impact of melatonin against age-dependent chronic arsenic toxicity in rats’ brains. J Exp Zool A Ecol Integr Physiol 337:1010–1024
Durappanavar PN, Nadoor P, Waghe P et al (2019) Melatonin ameliorates neuropharmacological and neurobiochemical alterations induced by subchronic exposure to arsenic in wistar rats. Biol Trace Elem Res 190:124–139. https://doi.org/10.1007/s12011-018-1537-1
Tolins M, Ruchirawat M, Landrigan P (2014) The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health 80:303–314. https://doi.org/10.1016/j.aogh.2014.09.005
Dutta S, Saha S, Mahalanobish S et al (2018) Melatonin attenuates arsenic induced nephropathy via the regulation of oxidative stress and inflammatory signaling cascades in mice. Food Chem Toxicol 118:303–316
Ali T, Rehman SU, Shah FA, Kim MO (2018) Acute dose of melatonin via Nrf2 dependently prevents acute ethanol-induced neurotoxicity in the developing rodent brain. J Neuroinflammation 15:1–19
Sadek KM, Lebda MA, Abouzed TK (2019) The possible neuroprotective effects of melatonin in aluminum chloride-induced neurotoxicity via antioxidant pathway and Nrf2 signaling apart from metal chelation. Environ Sci Pollut Res 26:9174–9183
Shah SA, Khan M, Jo M et al (2017) Melatonin stimulates the SIRT 1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci Ther 23:33–44
Solmaz I, Gürkanlar D, Gökçil Z et al (2009) Antiepileptic activity of melatonin in guinea pigs with pentylenetetrazol-induced seizures. Neurol Res 31:989–995
Kundurovic Z, Sofic E (2006) The effects of exogenous melatonin on the morphology of thyrocytes in pinealectomized and irradiated rats. J Neural Transm 113:49–58
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Graham DI (1977) Pathology of hypoxic brain damage in man. J Clin Pathol Suppl (R Coll Pathol) 11:170
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Mehri S, Meshki MA, Hosseinzadeh H (2015) Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug Chem Toxicol 38:162–166
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Xu S, He M, Zhong M et al (2015) The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neurosci Lett 590:52–57
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 3:71
Goudarzi M, Amiri S, Nesari A et al (2018) The possible neuroprotective effect of ellagic acid on sodium arsenate-induced neurotoxicity in rats. Life Sci 198:38–45. https://doi.org/10.1016/j.lfs.2018.02.022
Taheri Zadeh Z, Esmaeilpour K, Aminzadeh A et al (2021) Resveratrol attenuates learning, memory, and social interaction impairments in rats exposed to arsenic. Biomed Res Int 2021
Rudnitskaya EA, Muraleva NA, Maksimova KY et al (2015) Melatonin attenuates memory impairment, amyloid-β accumulation, and neurodegeneration in a rat model of sporadic Alzheimer’s disease. J Alzheimers Dis 47:103–116
Tyler CR, Allan AM (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 1:132–147
Du X, Tian M, Wang X et al (2018) Cortex and hippocampus DNA epigenetic response to a long-term arsenic exposure via drinking water. Environ Pollut 234:590–600
Ince S, Kucukkurt I, Turkmen R et al (2013) Dietary Yucca schidigera supplementation reduces arsenic-induced oxidative stress in Swiss albino mice. Toxicol Ind Health 29:904–914
Matsubara E, Bryant-Thomas T, Pacheco Quinto J et al (2003) Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem 85:1101–1108
Rahman MA, Hannan MA, Uddin MJ et al (2021) Exposure to environmental arsenic and emerging risk of Alzheimer’s disease: perspective mechanisms, management strategy, and future directions. Toxics 9:188
Omeiza NA, Abdulrahim HA, Alagbonsi AI et al (2021) Melatonin salvages lead-induced neuro-cognitive shutdown, anxiety, and depressive-like symptoms via oxido-inflammatory and cholinergic mechanisms. Brain Behav 11:e2227
Gonçalves JF, Fiorenza AM, Spanevello RM et al (2010) N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 186:53–60
Gu F, Chauhan V, Chauhan A (2015) Glutathione redox imbalance in brain disorders. Curr Opin Clin Nutr Metab Care 18:89–95. https://doi.org/10.1097/MCO.0000000000000134
Valdovinos-Flores C, Gonsebatt ME (2013) Nerve growth factor exhibits an antioxidant and an autocrine activity in mouse liver that is modulated by buthionine sulfoximine, arsenic, and acetaminophen. Free Radic Res 47:404–412. https://doi.org/10.3109/10715762.2013.783210
Zhou C, Zhao L, Zheng J et al (2017) MicroRNA-144 modulates oxidative stress tolerance in SH-SY5Y cells by regulating nuclear factor erythroid 2-related factor 2-glutathione axis. Neurosci Lett 655:21–27
Suh JH, Shenvi SV, Dixon BM et al (2004) Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci 101:3381–3386
Paladino S, Conte A, Caggiano R et al (2018) Nrf2 pathway in age-related neurological disorders: insights into MicroRNAs. Cell Physiol Biochem 47:1951–1976
Herskovits AZ, Guarente L (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81:471–483. https://doi.org/10.1016/j.neuron.2014.01.028
Davies DA, Adlimoghaddam A, Albensi BC (2021) Role of Nrf2 in synaptic plasticity and memory in Alzheimer’s disease. Cells 10. https://doi.org/10.3390/cells10081884
Yang D, Tan X, Lv Z et al (2016) Regulation of Sirt1/Nrf2/TNF-α signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci Rep 6:37157
Zhang Y, Duan X, Li J et al (2016) Inorganic arsenic induces NRF2-regulated antioxidant defenses in both cerebral cortex and hippocampus in vivo. Neurochem Res 41:2119–2128
He Z, Zhang Y, Zhang H et al (2021) NAC antagonizes arsenic-induced neurotoxicity through TMEM179 by inhibiting oxidative stress in Oli-neu cells. Ecotoxicol Environ Saf 223:112554
Firdaus F, Zafeer MF, Anis E et al (2018) Ellagic acid attenuates arsenic induced neuro-inflammation and mitochondrial dysfunction associated apoptosis. Toxicol Rep 5:411–417. https://doi.org/10.1016/j.toxrep.2018.02.017
Chaudhary S, Sahu U, Parvez S (2021) Melatonin attenuates branch chain fatty acid induced apoptosis mediated neurodegeneration. Environ Toxicol 36:491–505
Balarastaghi S, Barangi S, Hosseinzadeh H et al (2022) Melatonin improves arsenic-induced hypertension through the inactivation of the Sirt1/autophagy pathway in rat. Biomed Pharmacother 151:113135
Selvendiran K, Koga H, Ueno T et al (2006) Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res 66:4826–4834
Lau A, Whitman SA, Jaramillo MC, Zhang DD (2013) Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. J Biochem Mol Toxicol 27:99–105. https://doi.org/10.1002/jbt.21463
Do MT, Kim HG, Choi JH, Jeong HG (2014) Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med 74:21–34
Jiang W, Zhang X, Hao J et al (2014) SIRT1 protects against apoptosis by promoting autophagy in degenerative human disc nucleus pulposus cells. Sci Rep 4:7456
Zhu H, Lin Y, Liu Y (2021) miR-34a increases inflammation and oxidative stress levels in patients with necrotizing enterocolitis by downregulating SIRT1 expression. Mol Med Rep 24:1–9
Huang K, Huang J, Xie X et al (2013) Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med 65:528–540
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci 105:13421–13426
Wang L, Sun M, Cao Y et al (2020) miR-34a regulates lipid metabolism by targeting SIRT1 in non-alcoholic fatty liver disease with iron overload. Arch Biochem Biophys 695:108642
Ong C-S, Zhou J, Ong C-N, Shen H-M (2010) Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells via the Akt–GSK-3β–Cyclin D1 pathway. Cancer Lett 298:167–175
Kwon E-Y, Jung UJ, Park T et al (2015) Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes 64:1658–1669
Lin C, Zhao X, Sun D et al (2016) Transcriptional activation of follistatin by Nrf2 protects pulmonary epithelial cells against silica nanoparticle-induced oxidative stress. Sci Rep 6:21133
Wirawan E, Vande Walle L, Kersse K et al (2010) Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis 1:e18–e18
Marquez RT, Xu L (2012) Bcl-2: Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res 2:214
Fang S, Wan X, Zou X et al (2021) Arsenic trioxide induces macrophage autophagy and atheroprotection by regulating ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell Death Dis 12:88
Pi Q-Z, Wang X-W, Jian Z-L et al (2021) Melatonin alleviates cardiac dysfunction via increasing sirt1-mediated beclin-1 deacetylation and autophagy during sepsis. Inflammation 44:1184–1193
Funding
This work was funded by Mashhad University of Medical Science, Mashhad, Iran.
Author information
Authors and Affiliations
Contributions
Conceptualization: Gholamreza Karimi and Soghra Mehri; methodology: Gholamreza Karimi, Soghra Mehri, Nahid Najafi, Samira Barangi, Zahra Moosavi, and Seyed Hamid Aghaee-Bakhtiari; software: Nahid Najafi and Samira Barangi; investigation: Nahid Najafi; data curation: Nahid Najafi; writing—original draft and writing—review and editing: Nahid Najafi, Gholamreza Karimi, and Soghra Mehri; supervision and project administration: Gholamreza Karimi and Soghra Mehri; funding acquisition: Gholamreza Karimi. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article contained a mistake. Figure 2a in the original version of this article has been replaced.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Najafi, N., Barangi, S., Moosavi, Z. et al. Melatonin Attenuates Arsenic-Induced Neurotoxicity in Rats Through the Regulation of miR-34a/miR-144 in Sirt1/Nrf2 Pathway. Biol Trace Elem Res 202, 3163–3179 (2024). https://doi.org/10.1007/s12011-023-03897-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03897-5