Abstract
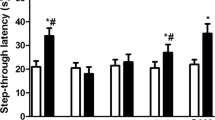
An experimental study was conducted in Wistar rats to characterize the arsenic (“As”)-induced alterations in neurobiochemistry in brain and its impact on neuropharmacological activities with or without the melatonin (MLT) as an antioxidant given exogenously. Male Wistar rats were randomly divided in to four groups of six each. Group I served as untreated control, while group II received As [sodium (meta) arsenite; NaAsO2] at 10 mg/kg bw (p.o.) for a period of 56 days. Experimental rats in group III received treatment similar to group II but in addition received MLT at 10 mg/kg bw (p.o.) from day 32 onwards. Rats in group IV received MLT alone from day 32 onwards similar to group III. Sub-chronic exposure to As (group II) significantly reduced both voluntary locomotor and forced motor activities and melatonin supplementation (group III) showed a significant improvement in motor activities, when subjected to test on day 42 or 56. Rats exposed to As showed a significant increase in anxiety level and a marginal nonsignificant reduction in pain latency. Sub-chronic administration of As induced (group II) significant increase in the levels of thiobarbituric acid reactive substance (TBARS) called malondialdehyde (MDA) in the brain tissue (5.55 ± 0.57 nmol g−1), and their levels were significantly reduced by MLT supplementation (group III 3.96 ± 0.15 nmol g−1). The increase in 3-nitrotyrosine (3-NT) levels in As-exposed rats indicated nitrosative stress due to the formation of peroxynitrite (ONOO−). However, exogenously given MLT significantly reduced the 3-NT formation as well as prostaglandin (PGE2) levels in the brain. Similarly, MLT administration have suppressed the release of pro-inflammatory cytokines (viz., IL-1β, IL-6, and TNF-α) and amyloid-β1–40 (Aβ) deposition in the brain tissues of experimental rats. To conclude, exogenous administration of melatonin can overcome the sub-chronic As-induced oxidative and nitrosative stress in the CNS, suppressed pro-inflammatory cytokines, and restored certain disturbed neuropharmacological activities in Wistar rats.









Similar content being viewed by others
References
Gulledge JH, O’connor JT (1973) Removal of arsenic (V) from water by adsorptioin on aluminum and ferric hydroxides, water technology/quality. J Am Water Works Assoc 65(8):548–552
Khan MA, Ho Y (2011) Arsenic in drinking water: a review on toxicological effects, mechanism of accumulation and remediation. Asian J Chem 23(5):1889–1901
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107
WHO (2011) Guideline for drinking-water quality, 4th edn. WHO press, Geneva
US-EPA (2001) Drinking water standards for arsenic. Office of Water. EPA- 815-F-00-015
IS 10500:2012 (2012) Indian standard drinking water specifications, Rev 2nd edn. Bureau of Indian Standards Publication, New Dehli, pp 1–16
Kim KW, Chanpiwat P, Hanh HT, Phan K, Sthiannopkao S (2011) Arsenic geochemistry of groundwater in Southeast Asia. Front Med 5(4):420–433
Akter KF, Owens G, Davey DE, Naidu R (2005) Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol 84:97–149
Namgung U, Xia Z (2000) Arsenite-induced apoptosis in cortical neurons is mediated by c-Jun N-terminal protein kinase 3 and p38 mitogen activated protein kinase. J Neurosci 20:6442–6451
Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37(12):1921–1942
Felix K, Manna SK, Wise K, Barr J, Ramesh GT (2005) Low levels of arsenite activates nuclear factor-kappa B and activator protein-1 in immortalized mesencephalic cells. J Biochem Mol Toxicol 19(2):67–77
Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD (2006) Methylated arsenicals: the implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit Rev Toxicol 36:99–133
Gurr JR, Liu F, Lynn S, Jan KY (1998) Calcium-dependent nitric oxide production is involved in arsenite-induced micronuclei. Mutat Res 416(3):137–148
Gandhi DN, Rakesh K (2013) Arsenic toxicity and neurobehaviors: A review. Inn Pharma Pharmacother 1(1):1–15
Stea F, Bianchi F, Cori L, Sicari R (2014) Cardiovascular effects of arsenic: clinical and epidemiological findings. Environ Sci Pollut Res Int 21:244–251
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH (2004) Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 112(13):1329–1333
Ashraf S, Ismail Z (2015) Chronic sodium arsenite exposure effects cognitive behaviour of Sprague Dawley rats. Am J Mater Sci 5:21–24
Flora SJ, Bhatt K, Mehta A (2009) Arsenic moiety in gallium arsenide is responsible for neuronal apoptosis and behavioral alterations in rats. Toxicol Appl Pharmacol 240(2):236–244
Liu X, Piao F, Li Y (2013) Protective effect of taurine on the decreased biogenic amine neurotransmitter levels in the brain of mice exposed to arsenic. Adv Exp Med Biol 776:277–287
Chandravanshi LP, Gupta R, Shukla RK (2018) Arsenic induced neurotoxicity by dysfunctioning cholinergic and dopaminergic system in brainof developing rats. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1452-5
Chang CY, Guo HR, Tsai WC, Yang KL, Lin LC, Cheng TJ, Chuu JJ (2015) Sub-chronic arsenic exposure enhances depression like behaviors in the reserpine induced mouse model of depression. Biomed Res Int 2015:159015
Maestroni GJ (1998) The photoperiod transducer melatonin and the immune-hematopoietic system. J Photochem Photobiol 43:186–192
Reiter RJ (1991) Melatonin: the chemical expression of darkness. Mol Cell Endocrinol 79:153–158
Reiter RJ (1991) Pineal melatonin: cell biology of its synthesis and its physiological interactions. Endocr Rev 12:151–180
Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2(2):181–197
Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z (2007) Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol 54(1):1–9
Hardeland R, Reiter RJ, Poeggeler B, Tan DX (1993) The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci Biobehav Rev 17:347–357
Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36(1):1–9
Urata Y, Honma S, Goto S, Todoroki S, Iida T, Cho S, Honma K, Kondo T (1999) Melatonin induces gammaglutamyl cysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic Biol Med 27:838–847
Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ (2003) Oxidative damage to catalase induced by peroxyl radicals: functional protection by melatonin and other antioxidants. Free Radic Res 37:543–553
Patrick L (2003) Toxic metals and antioxidants: part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev 8(2):106–128
Amer SAM, Al-harbi MS, Al-zahrani YAA (2016) Protective role of some antioxidants on arsenic toxicity in male mice: physiological and histopathological perspectives. Biol Med 8(1):1–8
Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F (1994) Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci 55:271–276
Mohamed EA (2015) The protective effect of melatonin vs. vitamin E in the ischemic/reperfused skeletal muscle in the adult male rat model. J Cytol Histol S3:005. https://doi.org/10.4172/2157-7099
Montilla-Lopez P, Munoz-Agueda MC, Feijoo Lopez M, Munoz-Castaneda JR, Bujalance-Arenas I, Túnez-Fiñana I (2002) Comparison of melatonin versus vitamin C on oxidative stress and antioxidant enzyme activity in Alzheimer's disease induced by okadaic acid in neuroblastoma cells. Eur J Pharmacol 451(3):237–243
Shaker ME, Houssen ME, Abo-Hashem EM, Ibrahim TM (2009) Comparison of vitamin E, L-carnitine and melatonin in ameliorating carbon tetrachloride and diabetes induced hepatic oxidative stress. J Physiol Biochem 65(3):225–233
Rao MV, Purohit AR (2011) Neuroprotection by melatonin on mercury induced toxicity in the rat brain. Pharmacol Pharm 2:375–385
Uygur R, Aktas C, Caglar V, Uygur E, Erdogan H, Ozen OA (2013) Protective effects of melatonin against arsenic-induced apoptosis and oxidative stress in rat testes. Toxicol Ind Health 32(5):848–859
Olivieri G, Hess C, Savaskan E, Ly C, Meier F, Baysang G, Brockhaus M, Müller-Spahn F (2001) Melatonin protects SHSY5Y neuroblastoma cells from cobalt-induced oxidative stress, neurotoxicity and increased β-amyloid secretion. J Pineal Res 31:320–325
Lin AM, Fang SF, Chao PL, Yang CH (2007) Melatonin attenuates arsenite-inducedapoptosis in rat brain: involvement of mitochondrial andendoplasmic reticulum pathways and aggregation of alpha-synuclein. J Pineal Res 43(2):163–171
Song J, Kang SM, Lee KM, Lee JE (2015) The protective effect of melatonin on neural stem cell against LPS-induced inflammation. Biomed Res Int 1–13. https://doi.org/10.1155/2015/854359
Kulkarni SK (1999) Hand book of experimental pharmacology, 3rd edn. Vallabh Prakashan, Delhi, pp 117–118
Dunham MW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Sci 46(3):208–209
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Turner RA (1965) Analgesic. In: Screening methods in pharmacology. Academic Press, New York, p 158
Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA (1998) Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res 82:810–818
Paula FB, Gouvea CM, Alfredo PP, Salgado I (2005) Protective action of a hexane crude extract of Pterodon emarginatus fruits against oxidative and nitrosative stress induced by acute exercise in rats. BMC Compliment Alternat Med 5:17–25
Madesh M, Balasubramanian KA (1998) Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys 35:84–188
Aebi HE (1983) Catalase. In: Bergmeyer HU, Bergmeyer J, Grabi M (eds) Methods of enzymatic analysis, vol III, third edn. Verlag Chemie, Weinheim, pp 273–286
Goldberg DM, Spooner RJ (1983) Glutathione reductase. In: Bergmeyer HU, Bergmeyer J, Grabi M (eds) Methods of enzymatic analysis, vol III. Verlag Chemie, Weinheim, pp 258–265
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound and nonprotein bound sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Waghe P, Sarath TS, Gupta P, Kutty HS, Kandasamy K, Mishra SK, Sarkar SN (2014) Subchronic arsenic exposure through drinking water alters vascular redox homeostasis and affects physical health in rats. Biol Trace Elem Res 162(1–3):234–241
Hasan M, Awal MA, Ara A, Rashid MB, Azam MG, Ali MH (2015) Study on prevention of induced arsenic toxicity in rats by using spirulina and thankuni. Sch J App Med Sci 3(2):828–832
Shagirtha K, Muthumani M, Prabu SM (2011) Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur Rev Med Pharmacol Sci 15:1039–1050
Saha S, Sadhukhan P, Mahalanobish S, Dutta S, Sil PC (2018) Ameliorative role of genistein against age-dependent chronic arsenic toxicity in murine brains via the regulation of oxidative stress and inflammatory signaling cascades. J Nutr Biochem 55:26–40. https://doi.org/10.1016/j.jnutbio.2017.11.010
Rang HP, Dale MM, Retter JM, Flower RJ (2007) Rang and Dale’s pharmacology, 6th edn. Churchill Livingstone Publication, UK, pp 473–644
Schwarz PB, Peever JH (2011) Dopamine triggers skeletal muscle tone by activating D1-like receptors on somatic motoneurons. J Neurophysiol 106(3):1299–1309
Deng Y, Jiao C, Mi C, Xu B, Li Y, Wang F, Liu W, Xu Z (2015) Melatonin inhibits manganese-induced motor dysfunction and neuronal loss in mice: involvement of oxidative stress and dopaminergic neurodegeneration. Mol Neurobiol 51(1):68–88
Kim YS, Joo WS, Jin BK, Cho YH, Baik HH, Park CW (1998) Melatonin protects 6-OHDA-induced neuronal death of nigrostriatal dopaminergic system. Neuroreport 9(10):2387–2390
Nagaraja TN, Desiraju T (1993) Regional alterations in the levels of brain biogenic amines, glutamate, GABA, and GAD activity due to chronic consumption of inorganic arsenic in developing and adult rats. Bull Environ Contam Toxicol 50:100–107
Karimi S, Jahanshahi M, Golalipour MJ (2014) The effect of MDMA-induced anxiety on neuronal apoptosis in adult male rats’ hippocampus. Folia Biol 60(4):187–191
Aktar S, Jahan M, Alam S, Mohanto NC, Arefin A, Rahman A, Haque A, Himeno S, Hossain K, Saud ZA (2017) Individual and combined effects of arsenic and lead on behavioral and biochemical changes in mice. Biol Trace Elem 177(2):288–296
Golombek DA, Martini M, Cardinali DP (1993) Melatonin as an anxiolytic in rats: time dependence and interaction with the central GABAergic system. Eur J Pharmacol 237:231–236
Kalonia H, Kumar A (2007) Protective effect of melatonin on certain behavioral and biochemical alterations induced by sleep-deprivation in mice. Indian J Pharm 39(1):48–51
Niles LP, Pickering DS, Arciszewski MA (1987) Effects of chronic melatonin administration on GABA and diazepam binding in rat brain. J Neural Transm 70(1–2):117–1124
Aguirre-Banuelos P, Escudero-Lourdes C, Sanchez-Pena LC, Del Razo LM, Perez-Urizar J (2008) Inorganic arsenic exposure affects pain behavior and inflammatory response in rat. Toxicol Appl Pharmacol 229(3):374–385
Vijayakaran K, Kesavan M, Kannan K, Sankar P, Tandan SK, Sarkar SN (2014) Arsenic decreases antinociceptive activity of paracetamol: possible involvement of serotonergicand endocannabinoid receptors. Environ Toxicol Pharmacol 38:397–405
Yu C, Zhu C, Xu S, Cao X, Wu G (2000) The analgesic effects of peripheral and central administration of melatonin in rats. Eur J Pharmacol 403:49–53
Valko M, Morris H, Cronin MT (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ (1993) Transfer of arsenite from glutathione to dithiols: a model of interaction. Chem Res Toxicol 6:598–602
El-Demerdash FM, Yousef MI, Radwan FM (2009) Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol 47:249–254
Garcia-Chavez E, Jimenez I, Segura B, Del Razo LM (2006) Lipid oxidative damage and distribution of inorganic arsenic and its metabolites in the rat nervous system after arsenite exposure: influence of alpha tocopherol supplementation. Neurotoxicology 27(6):1024–1031
Ahsan H (2013) 3-Nitrotyrosine: a biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol 74(10):1392–1399
Ding X, Hiraku Y, Ma N, Kato T, Saito K, Nagahama M, Semba R, Kuribayashi K, Kawanishi S (2005) Inducible nitric oxide synthase-dependent DNA damage in mouse model of inflammatory bowel disease. Cancer Sci 96(3):157–163
Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD (2004) Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol 201(1):32–39
Turjanski A, Chaia ZD, Doctorovich F, Estrin D, Rosenstein R, Piro OE (2000) N-nitrosomelatonin. Acta Crystallogr C 56:682–683
Limson J, Nyokong T, Daya S (1998) The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study. J Pineal Res 24(1):15–21
El-Sokkary GH, Kamel ES, Reiter RJ (2003) Prophylactic effect of melatonin in reducing lead-induced neurotoxicity in the rat. Cell Mol Biol Lett 8:461–470
Bharti VK, Srivastava RS (2009) Fluoride-induced oxidative stress in rat’s brain and its amelioration by buffalo (Bubalus bubalis) pineal proteins and melatonin. Biol Trace Elem Res 130:131–140
Rao MV, Purohit A, Patel T (2010) Melatonin protection on mercury-exerted brain toxicity in the rat. Drug Chem Toxicol 33(2):209–216
Bharti VK, Srivastava RS, Sharma B, Malik JK (2012) Buffalo (Bubalus bubalis) epiphyseal proteins counteract arsenic-induced oxidative stress in brain, heart and liver of female rats. Biol Trace Elem Res 146:224–229
Huang CC, Lai CJ, Tsai MH, Wu YC, Chen KT, Jou MJ, Fu PI, Wu CH, Wei IH (2015) Effects of melatonin on the nitric oxide system and protein nitration in the hypobaric hypoxic rat hippocampus. BMC Neurosci 16:61
Zaazaa AM (2014) Protective role of the nigella sativa oil against arsenic-induced neurotoxicity in male rats. World J Pharm Res 3(2):1624–1636
Vijayakaran K, Kannan K, Kesavan M, Suresh S, Sankar P, Tandan SK, Sarkar SN (2014) Arsenic reduces the antipyretic activity of paracetamol in rats: modulation of brain COX-2 activity and CB1 receptor expression. Environ Toxicol Pharmacol 37:438–447
Karin M, Delhase M (1998) JNK or IKK, AP-1 or NF-kappa B, which are the targets for MEK kinase 1 action? Proc Natl Acad Sci U S A 95:9067–9069
Mohan N, Sadeghi K, Reiter RJ, Meltz ML (1995) The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochem Mol Biol Int 37(6):1063–1070
Jain A, Mehta VK, Chittora R, Mahdi AA, Bhatnagar M (2015) Melatonin ameliorates fluoride induced neurotoxicity in young rats: an in vivo evidence. Asian J Pharm Clin Res 8(4):164–167
Rosales-Corral S, Tan DX, Reiter RJ, Valdivia-Velazquez M, Martinez-Barboza G, Acosta-Martinez JP, Ortiz GG (2003) Orally administered melatonin reduces oxidative stress and proinflammatory cytokines induced by amyloid-β peptide in rat brain: a comparative, in vivo study versus vitamin C and E. J Pineal Res 35:80–84
Glabe CC (2005) Amyloid accumulation and pathogensis of Alzheimer’s disease: significance of monomeric, oligomeric and fibrillar Abeta. Subcell Biochem 38:167–177
Koropatnick J, Pearson-Sharpe J, Fisman M (1994) Arsenic treatment induces accumulation of proteins derived from amyloid precursor protein (APP) in rat liver and brain. Neurobiol Aging 15(1):66
Acknowledgments
The authors are thankful to the Dean, Veterinary College, Shivamogga 577 204, Karnataka, India, for necessary facilities to conduct the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Prior approval of the Institutional Animal Ethics Committee (IAEC) was obtained, vide No. VCS/IAEC/005/2015-16 dated 23.07.2016 to carry out the current investigation as per the guidelines of the Committee for Prevention of Cruelty and Supervision of Experiment on Animals (CPCSEA), New Delhi.
Conflict of Interest
The authors declare that they have no conflicts of interest
Rights and permissions
About this article
Cite this article
Durappanavar, P.N., Nadoor, P., Waghe, P. et al. Melatonin Ameliorates Neuropharmacological and Neurobiochemical Alterations Induced by Subchronic Exposure to Arsenic in Wistar Rats. Biol Trace Elem Res 190, 124–139 (2019). https://doi.org/10.1007/s12011-018-1537-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1537-1