Abstract
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disorder characterized by the accumulation of accumulated alpha-synuclein (α-Syn) in substantia nigra. Research has shown that selenium (Se) can protect neural cells through the actions of selenoproteins, including selenoprotein P (SelP) and selenoprotein S (SelS), which participate in endoplasmic reticulum-associated protein degradation (ERAD). In this study, we investigated the potential protective role of Se in a pre-clinical PD rat model.
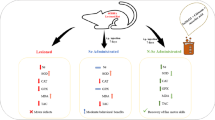
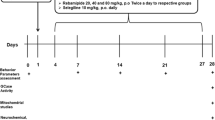
We aimed to evaluate the therapeutic effects of Se administration in the 6-hydroxydopamine (6-OHDA) induced unilateral rat PD model. Male Wistar rats were utilised for unilateral PD animal model which were subjected to stereotaxic surgery and injected with 20 μg 6-OHDA/5 μl 0.2% ascorbate saline. After confirming the model, the rats were intraperitoneally injected with 0.1, 0.2, and 0.3 mg/kg of sodium selenite for 7 days. We then performed behavioral tests, including apomorphine-induced rotation, hanging, and rotarod tests. Following sacrifice, we analysed the substantia nigra area of the brain and serum for protein quantification, element analysis, and gene expression analysis.
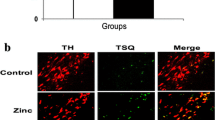
Our results indicate that the administration of 0.3 mg/kg of Se improved the motor deficiency in hanging, rotarod, and apomorphine-induced rotational tests. While there was no significant improvement in the expression of α-Syn, Se increased the expression of selenoproteins. Additionally, levels of selenoproteins, Se, and α-Syn both brain and serum were re-established by the treatment, suggesting the role of Se on the α-Syn accumulation. Furthermore, Se improved PD-induced biochemical deficits by increasing the levels of SelS and SelP (p<0.005).
In conclusion, our findings suggest that Se may have a protective role in PD. 0.3 mg/kg dosage of Se increased the expression of selenoproteins, reduced the accumulation of α-Syn in the brain, and improved PD-induced motor deficits. These results suggest that Se may be a potential therapeutic option for PD treatment.
Graphical abstract






Similar content being viewed by others
Data Availability
All generated or analyzed data during our research are included in this published article. Future data that support the findings are available from the corresponding author, upon reasonable requests.
Abbreviations
- α-Syn:
-
Alpha-synuclein
- ApoER2:
-
Apolipoprotein E receptors 2
- cDNA:
-
Complementary DNA
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- PD:
-
Parkinson’s disease
- PBS:
-
Phosphate-buffered saline
- Se:
-
Selenium/selenite sodium
- SelS:
-
Selenoprotein S
- SelP:
-
Selenoprotein P
- SEPS1/SES1:
-
Selenoprotein S gene
- SEPP1/SEP1:
-
Selenoprotein P gene
- SP/SeP:
-
Selenoproteins
- Sec:
-
Selenocysteine
- Se-Met:
-
Seleno-L-methionine
- SCNA:
-
α-Syn gene
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- i.p.:
-
Intraperitoneal
- ERAD:
-
Endoplasmic reticulum-associated protein degradation
- 6-OHDA:
-
6-Hydroxydopamine
- qRT-PCR:
-
Real-time quantitative PCR
- SNpc:
-
Substantia nigra pars compacta
- RDI:
-
Recommended daily intake
- MFB:
-
Medial forebrain bundle
- ER:
-
Endoplasmic reticulum
References
Nakajima A, Ohizumi Y (2019) Potential benefits of nobiletin, a citrus flavonoid, against Alzheimer’s disease and Parkinson’s disease. Int J Mol Sci 20(14):3380
Lo Bianco C et al (2002) α-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc Nat Acad Sci 99(16):10813–10818
Baquet ZC et al (2009) A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience 161(4):1082–1090
Balestrino R, Schapira A (2020) Parkinson disease. Eur J Neurol 27(1):27–42
Sherzai AZ et al (2016) Micronutrients and risk of Parkinson’s disease: a systematic review. Gerontol Geriatr Med 2:2333721416644286
Bianchi VE, Herrera PF, Laura R (2021) Effect of nutrition on neurodegenerative diseases. A Syst Rev Nutr Neurosci 24(10):810–834
Xu J et al (2020) Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages. Biol Trace Element Res 194:237–243
Laclaustra M et al (2010) Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis 210(2):643–648
Medicine, I.O (2000) Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids: a report of the panel on dietary antioxidants and related compounds, subcommittees on upper reference levels of nutrients. National Academies Press, Washington, DC
Bai K et al (2017) Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int J Nanomed 12:4527
Solovyev ND (2015) Importance of selenium and selenoprotein for brain function: from antioxidant protection to neuronal signalling. J Inorg Biochem 153:1–12
Saito Y (2021) Selenium transport mechanism via selenoprotein P—Its physiological role and related diseases. Front Nutr 8:685517
Saito Y et al (2004) Domain structure of bi-functional selenoprotein P. Biochem J 381(3):841–846
Shetty S, Copeland PR (2018) Molecular mechanism of selenoprotein P synthesis. Biochim Biophys Acta (BBA)-Gen Subj 1862(11):2506–2510
Seale LA et al (2018) Relationship between selenoprotein P and selenocysteine lyase: insights into selenium metabolism. Free Rad Biol Med 127:182–189
Torres DJ et al (2021) Selenoprotein P modulates methamphetamine enhancement of vesicular dopamine release in mouse nucleus accumbens via dopamine D2 receptors. Front Neurosci 15:631825
Bubenik JL, Miniard AC, Driscoll DM (2013) Alternative transcripts and 3′ UTR elements govern the incorporation of selenocysteine into selenoprotein S. PLoS One 8(4):e62102
Turanov AA et al (2014) Selenoprotein S is involved in maintenance and transport of multiprotein complexes. Biochem J 462(3):555–565
Shchedrina VA et al (2010) Structure–function relations, physiological roles, and evolution of mammalian ER-resident selenoproteins. Antiox Redox Sig 12(7):839–849
Lee JH et al (2014) Pro178 and Pro183 of selenoprotein S are essential residues for interaction with p97 (VCP) during endoplasmic reticulum-associated degradation. J Biol Chem 289(20):13758–13768
Dolatshahi M et al (2015) Ellagic acid improves hyperalgesia and cognitive deficiency in 6-hydroxidopamine induced rat model of Parkinson’s disease. Iran J Basic Med Sci 18(1):38
Atif F, Yousuf S, Agrawal SK (2008) Restraint stress-induced oxidative damage and its amelioration with selenium. Eur J Pharmacol 600(1-3):59–63
Zafar KS et al (2003) Dose-dependent protective effect of selenium in rat model of Parkinson’s disease: neurobehavioral and neurochemical evidences. J Neurochem 84(3):438–446
Haddadi H et al (2018) Chronic treatment with carvacrol improves passive avoidance memory in a rat model of Parkinson’s disease. Arquivos de neuro-psiquiatria 76:71–77
Kumari N et al (2018) Neuroprotective effect of IDPU (1-(7-imino-3-propyl-2, 3-dihydrothiazolo [4, 5-d] pyrimidin-6 (7H)-yl) urea) in 6-OHDA induced rodent model of hemiparkinson’s disease. Neurosci Lett 675:74–82
Haeri P et al (2019) Neuroprotective effect of crocin on substantia nigra in MPTP-induced Parkinson’s disease model of mice. Anatom Sci Int 94:119–127
Li Y, Hollis ER II (2023) Nicotinic acetylcholine signaling is required for motor learning but not for rehabilitation from spinal cord injury. Neural Regen Res 18(2):364
Younesian O et al (2022) Long-term excessive selenium supplementation affects gene expression in esophageal tissue of rats. Biol Trace Element Res 201(7):3387–3394
Lorestani S et al (2018) Increased glutathione reductase expression and activity in colorectal cancer tissue samples: an investigational study in Mashhad Iran. Middle East J Cancer 9(2):99–104
Wakabayashi K et al (2013) The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol Neurobiol 47:495–508
Blum D et al (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Progr Neurobiol 65(2):135–172
Thomas B et al (2011) Resistance to MPTP-neurotoxicity in α-synuclein knockout mice is complemented by human α-synuclein and associated with increased β-synuclein and Akt activation. PloS One 6(1):e16706
Quilty MC et al (2006) Alpha-synuclein is upregulated in neurones in response to chronic oxidative stress and is associated with neuroprotection. Exp Neurol 199(2):249–256
Chang C-W et al (2020) Plasma and serum alpha-synuclein as a biomarker of diagnosis in patients with Parkinson’s disease. Front Neurol 10:1388
Malek N et al (2014) Alpha-synuclein in peripheral tissues and body fluids as a biomarker for P arkinson’s disease–a systematic review. Acta Neurol Scand 130(2):59–72
Wakabayashi K (2020) Where and how alpha-synuclein pathology spreads in Parkinson’s disease. Neuropathology 40(5):415–425
Chandrasekhar Y et al (2018) Gallic acid protects 6-OHDA induced neurotoxicity by attenuating oxidative stress in human dopaminergic cell line. Neurochem Res 43:1150–1160
Xi Y et al (2018) MitoQ protects dopaminergic neurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1α. Biochim Biophys Acta (BBA)-Mol Basis Dis 1864(9):2859–2870
Yan T et al (2019) The neuroprotective effect of schisandrol A on 6-OHDA-induced PD mice may be related to PI3K/AKT and IKK/IκBα/NF-κB pathway. Exp Gerontol 128:110743
Jamal M et al (2022) COA-Cl evokes protective responses against H2O2-and 6-OHDA-induced toxic injury in PC12 cells. Neurotox Res 40(6):2061–2071
Monti B et al (2007) Alpha-synuclein protects cerebellar granule neurons against 6-hydroxydopamine-induced death. J Neurochem 103(2):518–530
Bourdenx M et al (2017) Protein aggregation and neurodegeneration in prototypical neurodegenerative diseases: examples of amyloidopathies, tauopathies and synucleinopathies. Progr Neurobiol 155:171–193
Sophiabadi M, Rastgoo N, Haghdoost-Yazdi H (2022) Dopaminergic neuronal death in substantia nigra associates with serum levels of total bilirubin, selenium, and zinc: evidences from 6-hydroxydopamine animal model of Parkinson’s disease. Biol Trace Element Res 200(9):4058–4067
Sampaio TB et al (2017) Involvement of BDNF/TrkB signaling in the effect of diphenyl diselenide on motor function in a Parkinson’s disease rat model. Eur J Pharmacol 795:28–35
Sun C et al (2023) Selenium forms and dosages determined their biological actions in mouse models of Parkinson’s disease. Nutrients 15(1):11
Rahbardar MG et al (2021) Protective effects of selenium on acrylamide-induced neurotoxicity and hepatotoxicity in rats. Iran J Basic Med Sci 24(8):1041
Ellwanger JH et al (2016) Biological functions of selenium and its potential influence on Parkinson’s disease. Anais da Acad Brasil Ciências 88:1655–1674
Khan HA (2010) Selenium partially reverses the depletion of striatal dopamine and its metabolites in MPTP-treated C57BL mice. Neurochem Int 57(5):489–491
Himeno S, Imura N (2002) Selenium in nutrition and toxicology. Heavy Metals in the Environment. Marcel Dekker Inc, New York, NY, pp 587–629
Vinceti M et al (2014) Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies. Toxicol Lett 230(2):295–303
Wei D et al (2015) Synthesis, characterization, antioxidant activity and neuroprotective effects of selenium polysaccharide from Radix hedysari. Carbohydr Polym 125:161–168
Bellinger FP et al (2012) Changes in selenoprotein P in substantia nigra and putamen in Parkinson's disease. J Parkinson's Dis 2(2):115–126
Zhang Z-H, Song G-L (2021) Roles of selenoproteins in brain function and the potential mechanism of selenium in Alzheimer’s disease. Front Neurosci 15:646518
Lee JH et al (2015) Selenoprotein S-dependent selenoprotein K binding to p97 (VCP) protein is essential for endoplasmic reticulum-associated degradation. J Biol Chem 290(50):29941–29952
Rueli RH et al (2017) Selenoprotein S reduces endoplasmic reticulum stress-induced phosphorylation of tau: potential role in selenate mitigation of tau pathology. J Alzheim Dis 55(2):749–762
Colla E (2019) Linking the endoplasmic reticulum to Parkinson’s disease and alpha-synucleinopathy. Front Neurosci 13:560
Kelly E et al (2009) Selenoprotein S/SEPS1 modifies endoplasmic reticulum stress in Z variant α1-antitrypsin deficiency. J Biol Chem 284(25):16891–16897
Remondelli P, Renna M (2017) The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Front Mol Neurosci 10:187
Abolarin PO et al (2022) Selenium reduces nociceptive response in acute 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced neurotoxicity. IBRO Neurosci Rep 12:1–11
Acknowledgements
The authors wish to extend special thanks to Kavosh laboratory for assisting with the expert advices to conducting experiments and Dr. Ali Shoeibi for his assistance.
Funding
This work was supported by Mashhad University of Medical Sciences under grant [number 980950]. Dr. Seyed Isaac Hashemy has received the grant.
Author information
Authors and Affiliations
Contributions
All of the authors contributed to the study conception. The study was designed by Dr. Seyed Isaac Hashemy. Material preparation was performed by Mahmoud Hosseini. Data collection and analysis were performed by Sanaz Salaramoli and Hamid Reza Joshaghani. The first draft of the manuscript was written by Sanaz Salaramoli and revised by Hamid Reza Joshaghani. All of the authors commented on previous versions of the manuscript. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Date of Research Proposal Approval
The research proposal was approved on 2020-07-22.
Ethics Approval
The Animal Research Ethics Committee of the Mashhad university of medical sciences accepted the protocols (Ethic number: IR.MUMS.MEDICAL.REC.1399.345).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Se reduces α-Syn accumulation.
2. Se induces SelP and SelS expression.
3. 6OHDA induces α-Syn accumulation in substantia nigra.
4. SelS alleviates α-Syn accumulation.
6. Se has therapeutic effects on PD.
7. Se improves PD-induced motor dysfunctions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salaramoli, S., Joshaghani, H.R., Hosseini, M. et al. Therapeutic Effects of Selenium on Alpha-Synuclein Accumulation in Substantia Nigra Pars Compacta in a Rat Model of Parkinson’s Disease: Behavioral and Biochemical Outcomes. Biol Trace Elem Res 202, 1115–1125 (2024). https://doi.org/10.1007/s12011-023-03748-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03748-3