Abstract
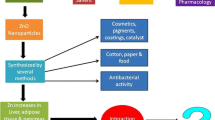
Zirconia nanoparticles are used in various industrial and biomedical applications such as dental implants, thermal barrier sprays, and fuel cells. The interaction of nanoparticles with the environment and humans is inevitable. Despite the enormous application potential of these nanoparticles, there are still some gaps in the literature regarding potential toxicological mechanisms and the genotoxicity of zirconia nanoparticles. The lung is one of the main exposure routes to nanomaterials; therefore, the present study was designed to determine the genotoxic and mutagenic effect of zirconia NPs in V-79 lung cells. Zirconia nanoparticles showed significant internalization in cells at 100 μg/mL and 150 μg/mL concentrations. Zirconia nanoparticles showed low cytotoxicity and were found to generate ROS in V-79 cells. In alkaline comet assay, zirconia nanoparticles (10 μg/mL, 50 μg/mL, and 100 μg/mL) exposed cells exhibited significant DNA strand breaks, while the neutral comet assay, which was used for double-strand break assessment, only revealed significant damage at 100 μg/mL. Chromosomal aberration induced by zirconia nanoparticles mainly resulted in the generation of gaps, few fragments, and breaks which signifies the low clastogenic activity of these nanoparticles in the V-79 cell line. In MN assay, zirconia nanoparticles resulted in no significant micronuclei induction at any given concentration. In the HPRT mutation assay, the particle shows a dose-dependent increase in the mutant frequency. It is evident from the result that zirconia nanoparticles cause dose-dependent cytotoxicity and genotoxicity, but still, more studies are needed to evaluate the clastogenic potential and the possible mechanism involved.






Similar content being viewed by others
References
Manicone P et al (2007) Biological considerations on the use of zirconia for dental devices. Inte J Immunopathol Pharmacol 20(1_suppl):9–12
Hafezeqoran A, Koodaryan R (2017) Effect of zirconia dental implant surfaces on bone integration: a systematic review and meta-analysis. BioMed Res Int 2017:9246721
Afzal A (2014) Implantable zirconia bioceramics for bone repair and replacement: a chronological review. Mater Express 4:1–12
Maiti TK, Majhi J, Maiti SK et al (2022) Zirconia- and ceria-based electrolytes for fuel cell applications: critical advancements toward sustainable and clean energy production. Environ Sci Pollut Res 29:64489–64512 . https://doi.org/10.1007/s11356-022-22087-9
Bakradze G (2011) Initial oxidation of zirconium: oxide-film growth kinetics and mechanisms
Bartter T et al (1991) Zirconium compound-induced pulmonary fibrosis. Arch Intern Med 151(6):1197–1201
Tuomi T (1993) Hypersensitivity Pneumonitis and Exposure to Zirconium Silicate in a young ceramic nle worker. Am Rev Respir Dis 148:1089–1092
Shin SW, Song IH, Um SH (2015) Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 5(3):1351–1365
Yazdanian M et al (2022) The potential application of green-synthesized metal nanoparticles in dentistry: a comprehensive review. Bioinorg Chem Appl 2022:2311910
Tanzi MC, Farè S, Candiani G (2019) Foundations of biomaterials engineering. Academic Press
Wang Z et al (2020) Oral intake of ZrO2 nanoparticles by pregnant mice results in nanoparticles’ deposition in fetal brains. Ecotoxicol Environ Saf 202:110884
Sun T et al (2021) Hepatic distribution and toxicity of zirconia nanoparticles in vivo and in vitro. Process Saf Environ Prot 147:134–145
Shang Y et al (2021) Zirconia nanoparticles induce Hela cell death through mitochondrial apoptosis and autophagy pathways mediated by ROS. Front Chem 9:522708
Asadpour E et al (2016) Oxidative stress-mediated cytotoxicity of zirconia nanoparticles on PC12 and N2a cells. J Nanopart Res 18(1):1–13
Ye M, Shi B (2018) Zirconia nanoparticles-induced toxic effects in osteoblast-like 3T3-E1 cells. Nanoscale Res Lett 13(1):1–12
Mehdikhani H, Aqababa H, Sadeghi L (2020) Effect of zirconium oxide nanoparticle on serum level of testosterone and spermatogenesis in the rat: an experimental study. Int J Reprod BioMedicine 18(9):765
Atalay H, Çeli̇k A, Ayaz F (2018) Investigation of genotoxic and apoptotic effects of zirconium oxide nanoparticles (20 nm) on L929 mouse fibroblast cell line. Chem Biol Interact 296:98–104
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Mosaddad SA et al (2020) Fabrication and properties of developed collagen/strontium-doped Bioglass scaffolds for bone tissue engineering. J Market Res 9(6):14799–14817
Repetto G, Del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3(7):1125–1131
Jain AK et al (2017) Impact of anatase titanium dioxide nanoparticles on mutagenic and genotoxic response in Chinese hamster lung fibroblast cells (V-79): the role of cellular uptake. Food Chem Toxicol 105:127–139
Nüsse M, Marx K (1997) Flow cytometric analysis of micronuclei in cell cultures and human lymphocytes: advantages and disadvantages. Mutat Res 392(1–2):109–115
Ghosh S, Sharma A, Talukder G (1992) Zirconium: an abnormal trace element in biology. Biol Trace Elem Res 35:247–271
Atik MD, Taylan A, Uçan ES (2022) Radiological findings in the case exposed to zirconium. Turk Thorac J 23(6):426
Carlson C et al (2008) Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B 112(43):13608–13619
Kose O et al (2020) Impact of the physicochemical features of TiO2 nanoparticles on their in vitro toxicity. Chem Res Toxicol 33(9):2324–2337
Behzadi S et al (2017) Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev 46(14):4218–4244
Kuhn DA et al (2014) Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol 5(1):1625–1636
Busch W et al (2011) Internalisation of engineered nanoparticles into mammalian cells in vitro: influence of cell type and particle properties. J Nanopart Res 13:293–310
Madhyastha H et al (2019) An opinion on nanomedicine and toxico-cellular crosstalk: considerations and caveats. Mater Today Proc 10:100–105
Długosz O et al (2020) Methods for reducing the toxicity of metal and metal oxide NPs as biomedicine. Materials 13(2):279
Yazdanian M et al (2020) Fabrication and properties of βTCP/zeolite/gelatin scaffold as developed scaffold in bone regeneration: in vitro and in vivo studies. Biocybernetics Biomed Eng 40(4):1626–1637
Fotakis G, Timbrell JA (2006) In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 160(2):171–177
Cudazzo G et al (2019) Lysosomotropic-related limitations of the BALB/c 3T3 cell-based neutral red uptake assay and an alternative testing approach for assessing e-liquid cytotoxicity. Toxicol In Vitro 61:104647
Xu H, Ren D (2015) Lysosomal physiology. Annu Rev Physiol 77:57–80
Anand SK et al (2020) Activation of autophagic flux via LKB1/AMPK/mTOR axis against xenoestrogen bisphenol-A exposure in primary rat hepatocytes. Food Chem Toxicol 141:111314
Palumbo P et al (2019) NOS2 inhibitor 1400W induces autophagic flux and influences extracellular vesicle profile in human glioblastoma U87MG cell line. Int J Mol Sci 20(12):3010
Mei Y et al (2013) Endoplasmic reticulum stress and related pathological processes. J Pharmacol Biomed Anal 1(2):1000107
Musgrove C, Camps M (2012) Models for detection of genotoxicity in vivo: present and future. Mutagenesis. Rijeka, Croatia: InTech: p. 31–50
Hoeijmakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411(6835):366–374
Bocchi C et al (2016) Characterization of urban aerosol: seasonal variation of mutagenicity and genotoxicity of PM2. 5, PM1 and semi-volatile organic compounds. Mutat Res 809:16–23
Bagatir G et al (2022) The effect of Anzer honey on X-ray induced genotoxicity in human lymphocytes: an in vitro study. Microsc Res Tech 85(6):2241–2250
Anderson D, Plewa MJ (1998) The international comet assay workshop. Oxford University Press and United Kingdom Environmental Mutagen Society
Hansen MF, Cavenee WK (1988) Tumor suppressors: recessive mutations that lead to cancer. Cell 53(2):173–174
Hussain A, Tebyaniyan H, Khayatan D (2022) The role of epigenetic in dental and oral regenerative medicine by different types of dental stem cells: a comprehensive overview. Stem Cells Int 2022:5304860
OECD (2016) Test No. 490: In vitro mammalian cell gene mutation tests using the thymidine kinase gene. OECD Publishing Paris, France
Luzhna L, Kathiria P, Kovalchuk O (2013) Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond. Front Genet 4:131
Acknowledgements
The authors are greatly thankful to Mr. J. Shankar, Dr. P.N. Saxena, and Ms. Divya Singh for their assistance in TEM, SEM, and XRD studies, respectively.
Funding
The authors acknowledge CSIR-Indian Institute of Toxicology Research (CSIR-IITR), Lucknow, and Council of Scientific and Industrial Research (CSIR), New Delhi, India, for funding. DM acknowledges the CSIR for the JRF and SRF funding. The institutional manuscript number is IITR/SEC/MS/2023/38.
Author information
Authors and Affiliations
Contributions
The work presented in this paper was done in collaboration with all authors. DM and AKP conceived and designed the experiments used in the paper. DM performed the experiments with the aid of RM and KD and SJ. KD and SJ contributed in data analysis of the experiments. DM wrote the manuscript of the paper with the help of AKP. All authors gave critical feedback and helped in research, analysis, and manuscript. AKP defined the research theme and supervised the findings of this work. All authors discussed the results and finalize manuscript and contributed in the approval of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mourya, D., Dubey, K., Jha, S. et al. In Vitro Effects of Zirconia Nanoparticles: Uptake, Genotoxicity, and Mutagenicity in V-79 cells. Biol Trace Elem Res 202, 927–940 (2024). https://doi.org/10.1007/s12011-023-03739-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03739-4