Abstract
The current study aimed to compare the utilization efficiency of iron (Fe) feed additives from either bulk or nano sources in Nile tilapia, Oreochromis niloticus diets on growth, haematological, immunity, anti-oxidative, and intestinal topography capacities. Five isonitrogenous and isoenergetic diets were performed; the basal diet served as a control with no Fe added, whereas the experimental diets were shaped by adding bulk-Fe2O3 and nano-Fe2O3 to the basal diet to preserve Fe levels at 0.2 and 0.4 mg kg−1, respectively. Results indicated that superior growth performance was recorded in fish-fed diets supplemented with 0.4 nano-Fe2O3 mg kg−1 diet. In addition, the highest (P ≤ 0.05) survival rate, absorption area of villous (AAV), mucosal to serosal amplification ratio (MSR), and villi parameters (height and width) were noticed in fish fed diet enrichment with either bulk or nano-Fe2O3 source. However, the superiority observed in nano-Fe2O3 fish groups. Also, the highest values of plasma albumin, total protein, high-density lipoprotein cholesterol (HDL-C), white blood cells (WBCs), and lymphocyte absolute count (LYM) (P ≤ 0.05) recorded in fish fed a diet supplemented with nano-Fe2O3 versus the basal diet. Moreover, the highest values of catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and plasma lysozyme activity (P ≤ 0.05) were observed in fish fed 0.4 mg/kg−1 nano-Fe2O3, while the lowest value was recorded in fish fed the control diet. The best value of malondialdehyde activity (P ≤ 0.05) recorded in a fish-fed diet supplemented with 0.4 mg/kg−1 nano-Fe2O3. The current findings emphasize the importance of including Fe to improve fish growth, immunity, antioxidant capabilities, and intestinal structure, primarily with a nano-Fe source, which demonstrated a more effective function in satisfying Nile tilapia dietary Fe requirements and improving the aforementioned parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, achieving food security through seafood production forces the farmers and aquaculture stakeholders towards intensification of tilapia’s production technique to secure the global demand for food fish with a global production of 6.5 million MT in 2018 [1, 2]. The sustainability of aquaculture production relies on many factors, such as the provision of balanced, complete diets that supply essential nutrients qualitatively and quantitatively to ensure maximum fish growth, control disease outbreaks and pathogen infections, and maintain health status at a high standard level [1–5,2,3,4,]. Iron is one of the essential micro-minerals and can maintain some physiological processes, including oxygen transport, fat oxidation, haemoglobin formation, stress tolerance, optimal enzyme activity, DNA synthesis, ATP production, electron transport, and improved immunological function [6, 7]. Iron deficiency has been associated with microcytic anaemia, growth depression, changes in intestinal morphology (villus width/length), immune suppression, haematological abnormalities, and disease susceptibility [8]. Fish can obtain the required iron either from the surrounding water via the gills or from their diet. However, the dietary source is considered the main route due to the limited absorption through the gills. As well as using dietary feed supplements of high availability are considered critical points should be followed during aquafeed formulation, to meet the nutritional demand, improve feed quality, and maximize the productivity of farmed fish [9, 10].
The evolution of nanotechnology science has resulted in different kinds of nanoparticles (NPs) that are necessarily used in biomedical sciences, agriculture, and industry [11]. Any particle having at least one dimension less than 100 nm is referred to as a nanoparticle, and as a result, its properties differ from those of their bulk materials [12]. In aquaculture, nanoparticles can reduce the prevalence and spread of pathogenic pathogens. [13] stated that some metal nanoparticles are capable of inhibiting the growth of several bacterial and fungal species, including freshwater cyanobacteria.
Iron supplements are available in different forms: organic, inorganic, and nanoparticles, varying in the degree of their bioavailability. It has been reported that nanoparticles have a higher absorption and utilization rate compared to other chemical forms, due to it has energy level, small size, active atoms content, and ability to pass cell membranes faster [14]. These causes explained the findings demonstrated previously by [8] in Rohu Labeo rohita fed iron nanoparticles. Thus, antioxidants, such as iron, have been added to farm animal feed to avoid deteriorative oxidative processes or to promote oxidative stability in order to maintain food quality and nutritional value [15].
Therefore, the present study investigated the efficacy of iron supplements from different sources (bulk-Fe2O3 and nano-Fe2O3) on performance, fillet nutrient compositions, haematological parameters, the blood biochemical profile, antioxidant enzymes, immune function, and the histomorphometry of the intestine of Nile tilapia.
Material and Methods
Experimental Diet Preparation
The basal diet (Diet 1; Table 1) was formulated to fulfil the requirements of Nile tilapia according to the [1]. Two sources of iron (Fe), the bulk-Fe2O3 and nano-Fe2O3, were added to the control diet at 0.2 (Diet 2) and 0.4 (Diet 3) mg kg−1. Also, nano-Fe2O3 was added to the control diet at 0.2 (Diet 4) and 0.4 (Diet 5) mg /kg−1 diet. Fe was supplemented from bulk (Fe2O3) and nanoscale sources. Nano-Fe2O3 (Sigma-Aldrich, 207,780–500: 99%, USA) was used as the Fe source. The sizes of the elemental nano-Fe particles are less than 73 μm. Bulk-Fe2O3 and nano-Fe2O3 were thoroughly mixed with the control diet; after that, 300 ml of water per kg of diet was added to form a dough. The feed mixture was pelleted (2-mm diameter die) via a laboratory pelletizer (CPM, California Pellet Mill Co., San Francisco, California, USA). The feed pellets were kept chilled at 4 °C until use. The proximate composition of the experimental diets nutrients was analyzed following [16] procedures. The dietary Fe contents were 124.72 (control), 124.92 (Diet 2), 125.12 (Diet 3), 124.92 (Diet 4), and 125.12 (Diet 5) mg kg−1 diet.
Experimental Protocol and Animal Care
Mono-sex Nile tilapia fingerlings were purchased from the private farm of Kafr El-Shaikh and adapted in central laboratory for Aquaculture Research, Abbassa, Abou-Hammad, Sharkia, Egypt, in two circular fiberglass tanks (1 m3 for each) for two weeks and fed the control diet containing 31.84% crude protein at a rate of 5% of the whole biomass three times daily before the start of the experiment. After adaptation, fish were fasted for 24 h. Four hundred and fifty healthy tilapias (9.10 ± 0.014 g) were randomly allocated to 15 tanks (80 × 50 × 50 cm; 200 L for each), representing the five groups with three replicates, 30 fish per aquarium. Fish fed at 3% of their body weight the experimental diets three times/day by hand for 12 weeks. Fish were weighed once every two weeks and the daily ration was attuned rendering to weight gain. During the experiment, water temperature was recorded daily with a mercury thermometer suspended at 15-cm depth. pH was determined by using a pH meter (Orion pH meter, Abilene, Texas, USA), while dissolved oxygen (mg/L) was measured using YSI model 56 oxygen meter (YSI Company, Yellow Springs Instrument, Yellow Springs, Ohio, USA). Total ammonia was measured using DREL/2 HACH kits (HACH Co., Loveland, Co. USA). During the feeding trial, the water quality parameters averaged (± standard deviation): Water temperature 26.8 ± 0.3 °C; dissolved oxygen 5.98 ± 0.22; pH values 8.17 ± 0.35; total ammonia 0.16 ± 0.01 mg/L. All tested water quality criteria (temperature, dissolved oxygen, pH value, and total ammonia) were within the acceptable limits for rearing Nile tilapia.
Growth and Body Indices Estimation
Initial body weight (g) (IBW) and final body weight (g) (FBW) of individual fish were recorded for all fish/each tank at the initiation and the termination of the experiment. Also, the number of fish in each tank was counted and recorded. Weight gain (WG) was calculated as follows: WG = FBW (g) − IBW (g); condition factor (K) was calculated using the following formula: K = (W/L3) × 100, where W = weight of fish in grams and L = total length of fish in cm; specific growth rate (SGR) = 100 × (Ln W2 − Ln W1)/T, where Ln = natural log, W1 = initial body weight, W2 = final body weight, and T = study period (84 days); feed conversion ratio (FCR) was calculated according to the equation: FCR = feed intake (g)/weight gain (g); protein efficiency ratio (PER) = weight gain (g)/protein ingested (g); survival rate percentage (SR) = 100 × (total number of fish at the end of the experiment / total number of fish at the start of the experiment) as described previously in [17] and renowned in the footnote of Table 2.
Sample Collection
At the end of the growth period, fish were deprived for 24 h and then anesthetized with tricaine methanesulfonate (MS222) at 150 mg/L [18]. Then, total number and weight of fish in each tank were recorded to calculate the final body weight, weight gain, and survival. Blood samples (500 micron) were taken from the caudal vein of three fish per each replicate by using 10% ethylenediaminetetraacetate (EDTA), then separated into two groups. The first blood group was separated to test the hematological parameters. While the second group of blood was centrifuged at 3000 g for 10 min, to get the blood plasma. The obtained plasma samples were saved at − 20 °C for further analysis. After blood collection, individual fish weight and length were recorded for later estimation of condition factor. Then, fish dissected, samples from anterior and posterior intestine were separated for histomorphometry determination. Intestinal samples were fixed in 10% neutral-buffered formalin until examination [19]. Additionally, other three fish from each replicate were anaesthetized by MS222 at 150 mg/L, homogenized, dried, and stored at − 20 °C for subsequent fish flesh proximate and Fe content analysis.
Sample Analysis
Blood Assay
Hematocrit (%) was analyzed according to [20] procedures. Hemoglobin (Hb, g dL−1) was determined using hemoglobin kits (cat. no. KT-731), which is a standardized procedure of the cyanmethemoglobin method. The RBCs (× 1012 L−1) and WBCs (× 109 L−1) numbers were counted by the indirect method described by [21]. The differential counting of WBC was determined according to [22] method. Mean corpuscular volume (MCV, fl), mean corpuscular hemoglobin (pg) (MCH), and mean corpuscular hemoglobin concentration (MCHC) (g dL−1) were measured using methodology reported by [23]. Oxygen carrying capacity was calculated by multiplying the Hb content with 1.25 oxygen combining power of Hb g−1 [24].
Total protein and albumin (g dL−1) of blood plasma were analyzed following [25, 26]. While globulin (g dL−1) was calculated by withdrawing albumin from total protein according to [27]. The liver enzyme activity, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) (U L−1) were determined as stated by [20]. Plasma total cholesterol (mg dL−1), triglyceride (mg dL−1), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) (mg dL−1) were estimated according to [28].
Plasma Lysozyme Activity and Antioxidant Biomarkers
Plasma lysozyme activity was measured by using the turbidimetric approach according to [29]. The oxidative enzymes including catalase (CAT), superoxidase dismutase (SOD), glutathione peroxidase (GPx), and melanodialdehyde (MDA) activities were measured using according to [30]. The liver of three fish from each replicate were weighed, rinsed and grinded in glass homogenizer tubes with ice-cold saline (to 0.1 g of liver was added 0.9 mL saline, pH 7.0), and centrifuged at 3000 g for 10 min. The supernatant was collected for assays of SOD. SOD was measured using water-soluble tetrazolium salt as a superoxide detector and expressed as units per milligram protein. For CAT activity assay, a mixture of 2.5 ml of phosphate buffer (pH 7.0), 2 ml of H2O2 solution, and 0.5 ml of sample was added to each tube. The hydrogen peroxide (H2O2 30 mM) was used as a substrate and the decrease in H2O2 concentration at 22 °C was measured spectrophotometrically at 240 nm for 1 min and expressed as specific activities (U/g protein). MDA activity was determined according to [31]. GPx level was measured using diagnostic kits (Bio-diagnostics, Giza, Egypt) following the manufacturer’s instructions according to the method of [32].
Histomorphometry Examination of the Intestine
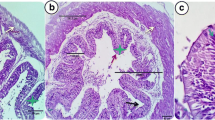
The histomorphometry parameters were determined by using a Rotatory Microtome (Reichert Technologies); the longitudinal and transverse slices, each 6 m thick, were cut and stained with haematoxylin and eosin in accordance with usual protocol. The light microscope supplied with a full HD microscopic camera and image processing software Olympus digital camera (Olympus LC20) was fixed on an Olympus microscope (Olympus BX-50) with a 1/2 × image adapter, and a × 40 objective was used to examine the tissue sections. Image analysis software was used to calculate the mean villus height (measured from the base to the top) for statistical analysis. The area of the absorption surface was determined as described by [32].
Fish Flesh Nutrient Composition
The proximate chemical analysis of fish fillet was analyzed following the technique revealed by [16]. Dry matter was measured after drying the samples in an oven (105 °C) for 24 h. Crude protein was analyzed by micro-Kjeldah method, N% × 6.25 (using Kjeltech auto analyzer, Model 1030; Tecator) and crude fat by Soxhlet extraction with diethyl ether (40–60° C). Ash was determined by ignition at 550 °C for 12 h. Fe concentrations in fish fillet were determined using atomic emission spectrophotometer (IRIS Advantage; Thermo Jarrell Ash Corporation) using standard Fe concentrations [16].
Statistical Analysis
Data generated are presented as mean ± standard error (SE), and were analyzed by using Statistical Analysis System, SAS [33] to identify the significant difference across various treatments, one-way analysis of variance (one-way ANOVA), and the [34] new multiple range test were applied. The data were found significant at P ≤ 0.05.
Results
Growth and Body Traits
Tilapia’s growth (FBW, WG, and SGR), feed utilization (FI and PER), and body survival rate markedly increased (P ≤ 0.05) in fish received diet supplied with Fe compared to basal diet (Table 2). The best (P ≤ 0.05) fish performance observed in tilapia group fed on 0.4 nano-Fe2O3 mg kg−1 diet.
Intestinal Morphometry
The morphometric indices of the intestine structures are illustrated in Table 3. Villi height and width measures in anterior and posterior intestine significantly (P < 0.05) increased by dietary supplementation of Fe, the highest significant (P < 0.05) measures recorded in nano-Fe2O3 fish groups. Absorption area of villous (AAV) of anterior intestine noticeably increased significant (P < 0.05) in fish fed 0.2 mg/kg−1 nano-Fe2O3, and in posterior intestine AAV and MSR values improved in fish fed nano-Fe2O3 at both levels 0.2 and 0.4 mg/kg−1. Mucosal to serosal amplification ratio (MSR) of anterior intestine significant (P < 0.05) rose in fish group received 0.4 mg/kg−1 nano-Fe2O3.
Haematological Analysis
Haematological parameters analysis is stated in Table 4. Hemoglobin concentration was the best (P ≤ 0.05) in fish received 0.4 nano-Fe2O3 mg kg−1 diet. Hematocrit percent improved (P ≤ 0.05) by Fe supplementation compared to the control free iron diet, whereas the highest (P ≤ 0.05) hematocrit percent noticed in those fed diet provided with nano-Fe2O3. Moreover, WBCs and LYM cell count considerably rose (P ≤ 0.05) by dietary addition of Fe compared to the control free iron diet, the best values measured in fish administered 0.2–0.4 mg kg−1 Nano-Fe supplemented diets compared to those fed other diets.
Plasma Biochemical Parameters
Plasma biochemical parameters are cleared in Table 5. Plasma ALT, AST, TC, TG, LDL-C, and VLDL-C levels noticeably reduced (P ≤ 0.05) by dietary supplementation of Fe, where the lowest significant values were in plasma of fish fed nano-Fe2O3. Furthermore, plasma albumin, total protein, and HDL-C demonstrated a considerable increase (P ≤ 0.05) in fish offered diet supplemented with Fe, where the highest values measured in fish received nano-Fe2O3.
Plasma Lysozyme Activity and Antioxidant Biomarkers
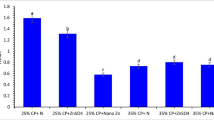
Plasma lysozyme activity and antioxidant indicators are displayed in Figs. 1, 2, and 3. Plasma lysozyme activity, catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) obviously rose (Fig. 1A and B and Fig. 2A and B) (P ≤ 0.05) in fish by dietary Fe inclusion, whereas the superior values reported in fish received 0.4 mg/kg−1 nano-Fe2O3 versus basal diet. The lowest value of MDA (P ≤ 0.05, Fig. 3) value was recorded in fish fed 0.4 mg/kg−1 nano-Fe2O3 versus other diets.
Fish Fillet Nutrient Composition
Fish received nano-Fe2O3 included diets revealed a decline (Table 6, P ≤ 0.05) in the moisture content at both level of inclusion. While fat fillet value reduced as dietary adding level of Fe increased either in bulk or nano source, whereas the lowest fillet fat value observed in fish received 0.4 nano-Fe2O3 mg kg−1 diet. In contrast, crude protein and ash levels increased in fillet of fish fed on diet supplied with Fe compared to the control diet, the highest significant (P ≤ 0.05) levels observed in fillet of fish offered the nano-Fe2O3 provided diets. The best value of fish fillet Fe contents considerably increased (P ≤ 0.05) in fish fed diet supplemented with 0.4 nano-Fe2O3.
Discussion
Growth and feed utilization markedly increased in tilapia received diet augmented with Fe versus to those fed the basal diet. The present study showed the best performance observed in fish groups fed on 0.4 Nano-Fe2O3 mg kg−1 diet. Besides, tilapia survival rate augmented significantly by inclusion of dietary addition of Fe either from bulk or nano source. The results agreed with Khan et al. [35] who observed that nano-nutrients complex (Zn, Se, Fe, and Cu) mixed with basal diet displayed the best growth traits; 33% higher final weight, better SGR and FCR values than feeding with a commercial basal diet. Similarly, [36] observed the elaboration effect of the diets containing iron nanoparticle on trout growth compared with the inorganic form. Such positive outcomes of nano iron diet recorded in tilapia [37].
Moreover, it has confirmed by [8] who found that basal diet provided with FeNPs improves the final weight of treated Labeo rohita. Similarly, improvement of growth and survival has been noticed in different fish species fed SeNP and ZnNP supplied diet [38, 39]. The present results could be attributed to the following reasons: (i) FeNPs ease nutrient assimilation that accordingly progress growth and feed efficiency [40], (ii) the introduction of FeNPs upsurge the specific surface area of compounds [41], (iii) higher bioavailability and utilization efficacy of of FeNPs compared to other forms of selenium [14, 42], (iv) the function of FeNPs as the nutrients trailer (especially AAs), these nutrients could pass into blood as a source of energy [41], (v) FeNPs construct as enzyme-cofactor indorse the breakdown and absorption of nutrients, (vii) FeNPs act as stimulatory and regulatory factor of bone formation and mineralization [43], (viii) FeNPs rapidly taken up by the cells, and exhibit higher bioavailability which accordingly enhance growth [42, 44], and (ix) the biological role of Fe as immunostimulant on bone formation, mineralization, and hematological parameters of fish [45, 46].
The number of goblet cells, villus width/length, MSR, and absorption area are important markers of intestinal morphology because they play a substantial part in nutritional absorption by extending and changing the absorption area of the fish intestine, resulting in better performance [47]. The intestinal topography as absorption area of villous (AAV) of anterior intestine noticeably increased in fish fed 0.2 mg kg−1 nano-Fe2O3, and in posterior intestine AAV and mucosal to serosal amplification ratio (MSR) values improved obviously in fish fed nano-Fe2O3 at both levels 0.2 and 0.4 mg kg−1. MSR of anterior intestine significantly rose in fish group received 0.4 mg kg.−1 nano-Fe2O3. The results indicate normal growth, health, and integrity of the intestinal structure. The amelioration of intestinal structure of tilapia due to iron supplementation particularly the nano form, which displayed the best measures, indicates that Fe is an essential trace element, and its dietary addition is required to meet the fish nutritional need and to perform its physiological function in regulating the normal tissues growth, immune, and the antioxidative defense and can protect from cell damage [8, 45, 46]
Haematological indices are displayed the physiological, immunity, and health status of fish, as well as disease and metabolic disorders [48–51,49,50,]. Also, leukocyte differentials act as the first line of defence against external invasions into the organism’s system [52]. The hematological parameters herein including: hemoglobin, hematocrit concentration, WBCs, and LYM cell count were the best in fish received nano-Fe2O3 mg kg−1. It confirms the biological role of Fe in the blood functions. The same amelioration effect of nano nutrients on the hematological parameters has been revealed by [37] who found the most haematological parameters, such as RBCs, hematocrit, and hemoglobin, were higher in trout fed nanoparticulate treatments than in the inorganic ones. Furthermore, [8] reported a substantial rise in the hematological parameters such as RBCs, hemoglobin of rohu fed the nano-Fe-fortified diets compared with the inorganic iron and the iron-deficient diet. The present results could be attributed to the following: (i) high availability of nano-Fe2O3 uptake on nanoparticle form. (ii) Iron is a chief constituent of RBCs [53]. Red cell indices (MCV, MCH, and MCHC) are key biomarkers that give helpful information on haemoglobin concentration and red blood cell sizes [49], and are therefore utilized to diagnose anaemia in animals (Yaji et al., 2018b). Changes (elevation and/or decrease) in red cell indices outside the normal physiological range usually indicate microcytic or macrocytic anaemia [54]. In the current data, there were insignificant differences in diets supplemented with either bulk-Fe or nano-Fe indicating normal physiological status of tilapia.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes in the serum of the blood are involved in cellular nitrogen metabolism, oxidation of amino acids, hepatic gluconeogenesis, and hepatic status, and their high levels in fish plasma could cause liver dysfunction [33, 55]. The lipid profile, which includes cholesterol and triglycerides, can change depending on nutritional status [56]. Triglycerides (TG) are measured to monitor lipid metabolism. High TG levels can cause glycogen storage disease, nephritic syndrome, and liver failure [57, 58]. The present study showed that fish fed nano-Fe2O3 recorded the lowest (P ≤ 0.05) values of ALT, AST, TC, TG, LDL-C, and VLDL-C levels compared to those fed control and bulk-Fe2O3. The current findings are in parallel with [37] who found positive effect of rainbow trout fed diets supplemented with nanoparticulate iron and copper on liver enzymes, blood biochemical parameters, antioxidant response, and immune function.
Furthermore, [38] noticed that inclusion of nano-Fe reduced the AST and ALT activities in Nile tilapia fish. The present results could be explained by the following: (i) the role of FeNPs in improving the fish health [46] and (ii) the role of FeNPs of decreasing the toxic and the stressful factors such as liver cell degeneration and necrosis which could increase the levels of ALT and AST [38, 46]. The present findings showed an improvement of plasma albumin, total protein, and HDL-C in fish offered diet supplemented with Fe, where the uppermost values measured in fish received nano-Fe2O3. Such effect has been noticed by [37] who detected those levels of serum total protein, albumin, and globulin contents in trout improved with Fe supplementation, where the highest values determined in fish fed with the nanoparticulate diets versus inorganic and control ones. Thus, confirming that minerals have beneficial in controlling the blood-protein synthesis in animals, and diets provided with minerals enhance the protein synthesis in fishes [7, 59, 60]. Also, it confirms that nano iron supplied diets were more potent in stimulation of blood protein synthesis. The increase of total protein level is linked with efficient protein utilization, increase in growth, and strong immunity due to the level of total protein gives evidence about the health, nutritional, and immune status [61, 62]. Also, albumin level improved in fish plasma fed nano iron indicating the higher ability of hepatocytes to synthesis the albumin and the efficient protein utilization. Likewise, [38] in tilapia and [62] in Clarias batrachus stated the effect of nano iron supplementation in increasing of blood proteins concentrations.
The lysozyme activity has a link with leucocytes and is produced mostly by macrophages, is the most important sign of the immune response as a result of many immune stimulants and microbiological components [63, 64]. The antioxidant enzymes revealed the body’s antioxidant system’s functional status, which reflected the body’s ability to break down oxygen-free radicals and to protect the tissues of the fish from oxidative damage. However, there is a link between antioxidant defense and fish responsiveness in aquaculture [65]. SOD is an antioxidant enzyme that catalyses the dismutation of superoxide anion into hydrogen peroxide and is therefore the first enzyme linked with the antioxidant defence line [66]. The GPx enzyme protects the animal against oxidative damage by converting hydroperoxides to alcohols [67]. Catalase activity plays a protective role against external superoxide sources by assuring the elimination of free hydrogen perioxide radicals. Lysozyme, CAT, GPX, and SOD obviously rose herein in fish by dietary Fe supplementation, whereas the uppermost values reported in fish received 0.4 mg kg−1 nano-Fe2O3 compared to fish fed other diets. The lowest value of MDA activity was recorded in fish fed 0.4 mgkg−1 nano-Fe2O3. The present results are in parallel with [37] who demonstrated that in general the addition of Fe improved the antioxidant enzymes and lysozyme activity, especially in the nanoscale form which revealed the highest values, compared with the bulk selenium and basal diets. The findings could be attributed to the following theories: (i) FeNPs decreasing the stressful conditions and promoting the innate immune parameters [46] and (ii) the present result highlight the importance of Nano technologies as an antioxidant component and quicken electron transfer. The present result of MDA assures the improved antioxidant defense of tilapia fed Fe supplied diets particularly in the nanoparticle form. Therefore, nano iron could be added to farm animal feed to avoid deteriorative oxidative processes or to promote oxidative stability in order to maintain food quality and nutritional value.
Fillet of fish received nano-Fe2O3 diets revealed an obvious decline in the moisture content at both level of inclusion compared to those fed the control and bulk-Fe2O3 diets. While values of fat in fillet reduced with increasing the inclusion levels of Fe either in bulk or nano source, whereas the lowest fillet fat value observed in fish received 0.4 nano-Fe2O3 mg kg−1 diet. The present results are in parallel with [36] who found that the fat and trans-fat contents of the fish fed diets contained nano-Fe2O3 were lower than those fed the basal diet. While the protein and ash levels increased in fillet of fish fed on diet supplied with Fe compared to the control diet, the highest significant values observed in fillet of fish fed nano-Fe2O3 diets. The current findings are in parallel with [36] who found that protein content in muscle of fish fed nano-nutrients higher than other groups. Fe content considerably increased in the fillet by dietary supplementation of Fe, whereas the best values recorded in fillet of fish fed nano-Fe2O3 mg kg−1. The present results are in parallel with [36] who found that high nutrients content of Fe in the muscles of fish fed with nano nutrients versus to those treated with a commercial diet. The current findings could be attributed to the following: (i) higher bioavailability of nano nutrients forms and (ii) nano-Fe2O3 has better connections with other substances and could be absorbed, retained, and reserved simply and more competently in the fish body [42–44,43,].
In conclusion, Fe is required to satisfy the nutritional needs of the fish as well as perform its physiological function in regulating proper tissue development, immunological and antioxidative defense, and cell damage protection. Thus, adding Fe in nano form to tilapia diets improves performance, immunological, fillet composition, and the healthiness of the intestinal structure more effectively than control-free Fe diets and bulk-Fe2O3 provided diets. However, further investigations for expression of some genes related to growth and antioxidant capacity are needed to clarify the function of nano-Fe2O3 on growth and health statues of fish.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
National Research Council (NRC) (2011). Nutrient requirements of fish and shrimp. Washington: DC: National Academy press
Stankus A (2021) State of world aquaculture 2020 and regional reviews: FAO webinar series. FAO Aquaculture Newsletter 63:17–18
Ogunji JO, Nimptsch J, Wiegand C, Schulz C, Rennert B (2011) Effect of housefly maggot meal (magmeal) diets on catalase, and glutathione S-transferase in the liver and gills of carp Cyprinus carpio fingerling. International Aquatic Research 3:11–20
Iheanacho SC, Ikwo N, Igweze NO, Chukwuidha C, Ogueji EO, Onyeneke R (2018) Effect of different dietary inclusion levels of melon seed (Citrullus lanatus) peel on growth, haematology and histology of Oroechromis niloticus juvenile. Turk J Fish Aquat Sci 18:377–384
Ogunji JO, Iheanacho SC, Abe GA, Ikeh OR (2020) Assessing effects of substituting dietary fish meal with boiled donkey and cow blood meal on growth performance and digestive enzyme activities of Clarias gariepinus juvenile. Journal of World Aquaculture Society 51:1066–1079
Antony Jesu Prabhu P, Schrama JW, Kaushik SJ (2016) Mineral requirements of fish: a systematic review. Rev Aquac 8(2):172–219
Kazemi E, Sourinejad I, Ghaedi A, Johari SA, Ghasemi Z (2020) Effect of different dietary zinc sources (mineral, nanoparticulate, and organic) on quantitative and qualitative semen attributes of rainbow trout (Oncorhynchus mykiss). Aquaculture 515:734529
Behera T, Swain P, Rangacharulu PV, Samanta M (2014) Nano-Fe as feed additive improves the hematological and immunological parameters of fish. Labeo rohita H Appl Nanosci 6:687–694
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151(1–4):185–207
Lall SP (2002) The minerals. In: Hardy RH, Halver JE (eds) Fish Nutrition, 3rd edn. Academic Press Elsevier Science, San Diego, CA, USA, pp 259–308
Płaza GA, Chojniak J, Banat IM (2014) Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int J Mol Sci 15(8):13720–13737
Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR (2009) Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol 10:634–641
Swain P, Nayak SK, Sasmal A, Behera T, Barik SK, Swain SK, Mishra SS, Sen AK, Das JK, Jayasankar P (2014) Antimicrobial activity of metal based nanoparticles against microbes associated with diseases in aquaculture. World J Microbiol Biotechnol 9:2491–2502
Wang H, Zhu H, Wang X, Li E, Du Z, Qin J, Chen L (2018) Comparison of copper bioavailability in copper-methionine, nano-copper oxide and copper sulfate additives in the diet of Russian sturgeon Acipenser gueldenstaedtii. Aquaculture 482:146–154
Min BR, Nam KC, Cordray JC, Ahn DU (2008) Factors Affecting Oxidative Stability of Pork, Beef, and Chicken Meat. Iowa State Univ Anim Ind Rep 5(1). https://doi.org/10.31274/ans_air-180814-1046
Lee M (1995) Official methods of analysis of AOAC International (16th edn): edited by Patricia A. Cunniff, AOAC International. $359.00 (North America)/$399.00 (elsewhere)(xxvi+ 1899 pages) ISBN 0 935 584 54 4. Elsevier
Ibrahim MS et al (2021) Nanoselenium versus bulk selenium as a dietary supplement: effects on growth, feed efficiency, intestinal histology, haemato-biochemical and oxidative stress biomarkers in Nile tilapia (Oreochromis niloticus Linnaeus, 1758) fingerlings. Aquac Res 52(11):5642–5655
EL-Erian MA, Ibrahim MS, Salem SMR, Mohammady EY, El-Haroun ER, Hassaan MS (2023) Evaluation of different copper sources in Nile tilapia diets: growth, body indices, hematological assay, plasma metabolites, immune, anti-oxidative ability, and intestinal morphometric measurements. Biol Trace Elem Res. https://doi.org/10.1007/s12011-023-03570-x
Caruso M, Demonte A, Neves VA (2012) Histomorphometric study of role of lactoferrin in atrophy of the intestinal mucosa of rats. Health 4(12):1362–1370
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Martins ML, Tavares-Dias M, Fujimoto RY, Onaka EM, Nomura DT (2004) Haematological alterations of Leporinus macrocephalus (Osteichthyes: Anostomidae) in fish pond. Arq Bras Med Vet Zool 56(5):640–646
Rosenfeld G (1947) Dye pancrômico for hematology and clinical cytology. New combinaҫӑo components of the May-Grünwald and Giemsa in one quick job coranate. Mem Inst Butantan 20:329–334
Lewis SM, Bain BJ, Bates ID (2001) Lewis Practical Hematology. 9 [sup], th. Churchill Livingstone, London, UK
Sampath K, James R, Akbar Ali KM (1998) Effects of copper and zinc on blood parameters and prediction of their recovery in Oreochromis mossambicus (pisces). Indian J Fish 45:129–139
Wotton I, Freeman H (1974) Microanalysis in medicinal biochemical. Churchill Livingstone, Edinburgh, London: p 1982
Coles MG (1974) Physiological activity and detection: The effects of attentional requirements and the prediction of performance. Biol Psychol 2:113–125
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin chem 18(6):499–502
Schäperclaus W, Kulow H, Schreckebach K (1992) Infectious abdominal dropsy. Fish diseases Berlin: AkademieVerlag 1:401–458
Ibrahim MS, El-Gendi GM, Ahmed AI, El-Haroun ER, Hassaan MS (2022) Nano zinc versus bulk zinc form as dietary supplied: effects on growth, intestinal enzymes and topography, and hemato-biochemical and oxidative stress biomarker in Nile tilapia (Oreochromis niloticus Linnaeus, 1758). Biol Trace Elem Res 200(3):1347–1360
Dogru MI, Dogru AK, Gul M, Esrefoglu M, Yurekli M, Erdogan S, Ates B (2008) The effect of adrenomedullin on rats exposed to lead. J Appl Toxicol 28(2):140–146
Moin VM (1986) A simple and specific method for determining glutathione peroxidase activity in erythrocytes. Lab Delo 12:724–727
Mohammady EY, Soaudy MR, Abdel-Rahman A, Abdel-Tawwabd M, Hassaan MS (2021) Comparative effects of dietary zinc forms on performance, immunity, and oxidative stress-related gene expression in Nile tilapia. Oreochromis niloticus Aquaculture 532:736006
SAS, 1993. Statistical Analysis System, SAS/STAT user Guide Release 6.03 Edition. SAS Institute Inc, Cary, North Carolina, USA
Duncan DB (1955) Multiple range and multiple F tests. Biom 11(1):1–42
Khan MZ, Hossain MM, Khan M, Ali MS, Aktar S, Moniruzzaman M, Khan M (2020) Influence of nanoparticle-based nano-nutrients on the growth performance and physiological parameters in tilapia (Oreochromis niloticus). RSC Adv 10(50):29918–29922
Afshari A, Sourinejad I, Gharaei A, Johari SA, Ghasemi Z (2021) The effects of diet supplementation with inorganic and nanoparticulate iron and copper on growth performance, blood biochemical parameters, antioxidant response and immune function of snow trout Schizothorax zarudnyi (Nikolskii, 1897). Aquaculture 539:736638
El-Shenawy AM, Gad DM, Yassin SA (2019) Effect of iron nanoparticles on the development of fish farm feeds. Alex J Vet Sci 60(1):102–115
Saravana MT, Bhavan P, Radhakrishnan S, Seenivasan C, Srinivasan V (2016) The effect of copper nanoparticles supplementation on freshwater prawn Macrobrachium rosenbergii post larvae. J Trace Elem Med Biol 34:39–49
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29
Zhou X, Wang Y, Gu Q, Li W (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291(1–2):78–81
Dawood MA, Zommara M, Eweedah NM, Helal AI (2020) The evaluation of growth performance, blood health, oxidative status and immune-related gene expression in Nile tilapia (Oreochromis niloticus) fed dietary nanoselenium spheres produced by lactic acid bacteria. Aquaculture 515:734571
Awad A, Zaglool AW, Ahmed SA, Khalil SR (2019) Transcriptomic profile change, immunological response and disease resistance of Oreochromis niloticus fed with conventional and Nano-Zinc oxide dietary supplements. Fish Shellfish Immunol 93:336–343
Cortinhas CS, Botaro BG, Sucupira MC, Renno FP, Santos MV (2010) Antioxidant enzymes and somatic cell count in dairy cows fed with organic source of zinc, copper and selenium. Livest sci 127(1):84–87
Beisel WR (1982) Single nutrients and immunity. Am J Clin Nutr 35:417–468
Andersen F, Lygren B, Maage A, Waagbø R (1998) Interaction between two dietary levels of iron and two forms of ascorbic acid and the effect on growth, antioxidant status and some non-specific immune parameters in Atlantic salmon (Salmo salar) smolts. Aquaculture 161(1–4):437–451
Wang J, Wang A, Wang W-X (2017) Evaluation of nano-ZnOs as a novel Zn source for marine fish: importance of digestive physiology. Nanotoxicology 11(8):1026–1039
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500:237–242
Ogueji EO, Nwani CD, Iheanacho SC, Mbah CE, Okeke CO, Yaji A (2018) Acute toxicity effects of ibuprofen on behaviour and haematological parameters of African catfish Clarias gariepinus (Burchell, 1822). Afr J Aquat Sci. https://doi.org/10.2989/16085914.2018.1465393
Hassaan MS, Mohammady EY, Soaudy, et al (2019) Effect of Silybum marianum seeds as a feed additive on growth performance, serum biochemical indices, antioxidant status, and gene expression of Nile tilapia, Oreochromis niloticus (L.) fingerling. Aquaculture 509:178–187
Hassaan MS, Nssar KM, Mohammady EY, Amin A, Tayel SI, El-Haroun ER (2020) Nano-zeolite efficiency to mitigate the aflatoxin B1 (AFB1) toxicity: effects on growth, digestive enzymes, antioxidant, DNA damage and bioaccumulation of AFB1 residues in Nile tilapia (Oreochromis niloticus). Aquaculture 523:735123
Jovanovi ć B, (2017) Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr Environ Assess Manag 13:510–515. https://doi.org/10.1002/ieam.191
Uzo-God OC, Agarwal A, Singh NB (2019) Effects of dietary nano and macro iron oxide (Fe2O3) on the growth, biochemical, and hematological profiles of African catfish (Clarias gariepinus) fingerlings. J Appl Aquac 31(2):153–171
Dacie JV, Lewis SM (2011) Practical Hematology, 11th edn. Churchill Livingstone, New York, p 41
Moazenzadeh K, Islami HR, Zamini A, Soltani M (2018) Effects of dietary zinc level on performance, zinc status, tissue composition and enzyme activities of juvenile Siberian sturgeon, Acipenser baerii (Brandt 1869). Aquac Nutr 24:1330–1339
Regost C, Arzel J, Cardinal M, Laroche M, Kaushik SJ (2001) Fat deposition and flesh quality in seawater reared, triploid brown trout (Salmo trutta) as affected by dietary fat levels and starvation. Aquaculture 193(3–4):325–345
Coz-Rakovac R, Strunjak-Perovic I, Hacmanjek M, Lipej Z, Sostaric B (2005) Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet Res Commun 29(8):677–687
Osman AG, Koutb M, Sayed AEDH (2010) Use of hematological parameters to assess the efficiency of quince (Cydonia oblonga Miller) leaf extract in alleviation of the effect of ultraviolet–a radiation on African catfish Clarias gariepinus (Burchell, 1822). J Photochem Photobiol, B 99(1):1–8
Chupani L, Niksirat H, Lünsmann V, Haange SB, von Bergen M, Jehmlich N, Zuskova E (2018) Insight into the modulation of intestinal proteome of juvenile common carp (Cyprinus carpio L.) after dietary exposure to ZnO nanoparticles. Sci Total Environ 613:62–71
Coeurdacier JL, Dutto G, Gasset E, Blancheton JP (2011) Is total serum protein a good indicator for welfare in reared sea bass (Dicentrarchus labrax)? Aquat Living Resour 24(2):121–127
Riche M (2007) Analysis of refractometry for determining total plasma protein in hybrid striped bass (Morone chrysops× M. saxatilis) at various salinities. Aquac 264(1–4):279–84
Akter N, Alam MJ, Jewel MA, Ayenuddin M, Haque SK, Akter S (2018) Evaluation of dietary metallic iron nanoparticles as feed additive for growth and physiology of Bagridae catfish Clarias batrachus (Linnaeus, 1758). Int J Fish Aquat Stud 6(3):371–377
Siwicki AK, Anderson DP (1993) Non-specific defence mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs. In: Siwicki AK, Anderson DP, Waluga J (eds) Disease diagnosis and prevention methods, FAO-project GCP/INT/526/JPN. IFI, Olsztyn, Poland, pp 105–111
Ringø E, Olsen RE, Vecino JG, Wadsworth S, Song S (2012) Use of immunostimulants and nucleotides in aquaculture: a review. J Mar Sci Res Dev 2:104
Guerriero G, Di Finizio A, Ciarcia G (2002) Stress-induced changes of plasma antioxidants in aquacultured sea bass, Dicentrarchus labrax. Comparative Biochem Physiol Part A 132:205–211
Song SB, Xu Y, Zhou BS (2006) Effects of hexachlorobenzene on antioxidant status of liver and brain of common carp (Cyprinus carpio). Chemistry 65:699–706
Ajima MNO, Pandey PK, Kumar K, Poojary N (2017) Neurotoxic, molecular responses and oxidative stress biomarkers in Nile tilapia, Oreochromis niloticus (Lin. 1758) exposed to verapmil. Comparative Biochemistry and Physiology Part C 196:44–52
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress894 biomarkers in marine organisms. Mar Environ Res 93:106–117
Acknowledgements
The authors acknowledge the Animal Production Department, Faculty of agriculture, Benha University, Cairo, Egypt, and National Institute of Oceanography and Fisheries (NIOF), Egypt, for assistance during this research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding is provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Eman Y. Mohammady: experiment design, collecting data, statistical analyses, and drafting the paper; Mohamed S. Ibrahim: statistical analyses and drafting the paper; Mohamed Elarian: collecting data and drafting the paper; Shimaa M.R. Salem: statistical analyses and drafting the paper; Ehab R. El-Haroun: drafting the paper; Mohamed S. Hassaan: drafting and edition the paper. All authors contributed to the study equally. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experiments were performed in accordance with relevant guidelines and regulations. Our reporting of research involving animals follows the recommendations of the ARRIVE guidelines. Approval was granted by the Animal Care and Use Committee at Mansura University (MU-ACUC; VM.R.23.02.57), Egypt.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammady, E.Y., Elashry, M.A., Ibrahim, M.S. et al. Nano Iron Versus Bulk Iron Forms as Functional Feed Additives: Growth, Body Indices, Hematological Assay, Plasma Metabolites, Immune, Anti-oxidative Ability, and Intestinal Morphometric Measurements of Nile tilapia, Oreochromis niloticus. Biol Trace Elem Res 202, 787–799 (2024). https://doi.org/10.1007/s12011-023-03708-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03708-x