Abstract
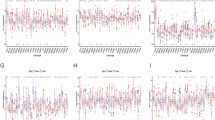
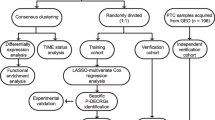
Current immunotherapy for prostate cancer is still in the stage of clinical trials. This delay is thought to be caused by an unclear regulatory mechanism of the immune microenvironment, which makes it impossible to distinguish patients suitable for immunotherapy. Cuprotosis may be related to the heterogeneity of immune microenvironment, which was regarded as a new copper-dependent cell death mode, was proposed, and gain attention. We explored for the first time the relationship between cuprotosis and the immune microenvironment of prostate cancer and constructed cuprotosis score. RNA sequencing data sets for prostate cancer were downloaded from public databases. Consensus clustering was applied to distinguish cuprotosis phenotype based on the expression of cuproptosis-related genes (CRGs) identified as prognostic factors. Genomic phenotypes of CRG clusters were depicted via consensus clustering. Cuprotosis score was established on the basis of differentially expressed genes (DEGs) identified as prognostic factors via principal component analysis. Cuprotosis score = the first principal component of prognostic factors + the second principal component of prognostic factors. The value of cuproptosis score in predicting prognosis and immunotherapy response was evaluated. PDHA1 (HR = 3.86, P < 0.001) and GLS (HR = 1.75, P = 0.018) were risk factors for prognosis of prostate cancer patients, while DBT (HR = 0.66, P = 0.048) was a favorable factor for prognosis of prostate cancer patients. CRG clusters had different prognosis and immune cell infiltration. So as gene clusters. Prostate cancer patients with low cuprotosis score showed better prognosis for biochemical relapse-free survival. Cuprotosis score is accompanied with high immune score and Gleason score. As cuprotosis genes, PDHA1, GLS, and DBT were identified as independent prognostic factors of prostate cancer. Cuprotosis score was established via principal component analysis of PDHA1, GLS, and DBT, which can be used as a predictor of prognosis and immunotherapy response of prostate cancer patients, and can characterize immune cells infiltration in tumors. Cuproptosis was involved in the regulation of immune microenvironment, which may depend on the effect of tricarboxylic acid cycle. Our study provided clues to reveal the relationship between copper death and immune microenvironment, highlighted the clinical significance of cuproptosis, and provided a reference for the development of personalized immunotherapy.






Similar content being viewed by others
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rebello RJ, Oing C, Knudsen KE et al (2021) Prostate cancer[J]. Nat Rev Dis Primers 7(1):9
Sandhu S, Moore CM, Chiong E et al (2021) Prostate cancer[J]. Lancet 398(10305):1075–1090
Achard V, Putora PM, Omlin A et al (2022) Metastatic prostate cancer: treatment options[J]. Oncology 100(1):48–59
Lowrance WT, Breau RH, Chou R et al (2021) Advanced prostate cancer: AUA/ASTRO/SUO guideline part I[J]. J Urol 205(1):14–21
Teo MY, Rathkopf DE, Kantoff P (2019) Treatment of advanced prostate cancer[J]. Annu Rev Med 70:479–499
Komura K, Sweeney CJ, Inamoto T et al (2018) Current treatment strategies for advanced prostate cancer[J]. Int J Urol 25(3):220–231
Cha HR, Lee JH, Ponnazhagan S (2020) Revisiting immunotherapy: a focus on prostate cancer[J]. Cancer Res 80(8):1615–1623
Isaacsson VP, Antonarakis ES (2018) PD-1/PD-L1 pathway inhibitors in advanced prostate cancer[J]. Expert Rev Clin Pharmacol 11(5):475–486
Abbott M, Ustoyev Y (2019) Cancer and the immune system: the history and background of immunotherapy[J]. Semin Oncol Nurs 35(5):150923
Zhang Y, Zhang Z (2020) The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications[J]. Cell Mol Immunol 17(8):807–821
Hong WX, Haebe S, Lee AS et al (2020) Intratumoral immunotherapy for early-stage solid tumors[J]. Clin Cancer Res 26(13):3091–3099
Chakravarty D, Huang L, Kahn M et al (2020) Immunotherapy for metastatic prostate cancer: current and emerging treatment options[J]. Urol Clin North Am 47(4):487–510
O’Donnell JS, Teng M, Smyth MJ (2019) Cancer immunoediting and resistance to T cell-based immunotherapy[J]. Nat Rev Clin Oncol 16(3):151–167
Tsaur I, Brandt MP, Juengel E et al (2021) Immunotherapy in prostate cancer: new horizon of hurdles and hopes[J]. World J Urol 39(5):1387–1403
Mitsogiannis I, Tzelves L, Dellis A et al (2022) Prostate cancer immunotherapy[J]. Expert Opin Biol Ther 22(5):577–590
Lei X, Lei Y, Li JK et al (2020) Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy[J]. Cancer Lett 470:126–133
Xiao J, Liu Z, Wang J et al (2022) Identification of cuprotosis-mediated subtypes, the development of a prognosis model, and influence immune microenvironment in hepatocellular carcinoma[J]. Front Oncol 12:941211
Xu Y, Li H, Lan A et al (2022) Cuprotosis-related genes: predicting prognosis and immunotherapy sensitivity in pancreatic cancer patients[J]. J Oncol 2022:2363043
Li Z, Zhang H, Wang X et al (2022) Identification of cuproptosis-related subtypes, characterization of tumor microenvironment infiltration, and development of a prognosis model in breast cancer[J]. Front Immunol 13:996836
Guo H, Wang Y, Cui H et al (2022) Copper induces spleen damage through modulation of oxidative stress, apoptosis, DNA damage, and inflammation[J]. Biol Trace Elem Res 200(2):669–677
Chen Y (2022) Identification and validation of cuproptosis-related prognostic signature and associated regulatory axis in uterine corpus endometrial carcinoma[J]. Front Genet 13:912037
Yuan H, Qin X, Wang J et al (2022) The cuproptosis-associated 13 gene signature as a robust predictor for outcome and response to immune- and targeted-therapies in clear cell renal cell carcinoma[J]. Front Immunol 13:971142
Liu J, Lu Y, Dai Y et al (2022) A comprehensive analysis and validation of cuproptosis-associated genes across cancers: overall survival, the tumor microenvironment, stemness scores, and drug sensitivity[J]. Front Genet 13:939956
Theophanides T, Anastassopoulou J (2002) Copper and carcinogenesis[J]. Crit Rev Oncol Hematol 42(1):57–64
Gaetke LM, Chow-Johnson HS, Chow CK (2014) Copper: toxicological relevance and mechanisms[J]. Arch Toxicol 88(11):1929–1938
Kahlson MA, Dixon SJ (2022) Copper-induced cell death[J]. Science 375(6586):1231–1232
Cobine PA, Brady DC (2022) Cuproptosis: cellular and molecular mechanisms underlying copper-induced cell death[J]. Mol Cell 82(10):1786–1787
Jiang Y, Huo Z, Qi X et al (2022) Copper-induced tumor cell death mechanisms and antitumor theragnostic applications of copper complexes[J]. Nanomedicine (Lond) 17(5):303–324
Krishnamoorthy L, Cotruvo JJ, Chan J et al (2016) Copper regulates cyclic-AMP-dependent lipolysis[J]. Nat Chem Biol 12(8):586–592
Collins CJ, Yi F, Dayuha R et al (2021) Direct measurement of ATP7B peptides is highly effective in the diagnosis of Wilson disease[J]. Gastroenterology 160(7):2367–2382
Squitti R, Ventriglia M, Simonelli I et al (2021) Copper imbalance in Alzheimer’s disease: meta-analysis of serum, plasma, and brain specimens, and replication study evaluating ATP7B gene variants. Biomolecules 11(7)
DiNicolantonio JJ, Mangan D, O’Keefe JH (2018) Copper deficiency may be a leading cause of ischaemic heart disease[J]. Open Heart 5(2):e784
Schepisi G, Cursano MC, Casadei C et al (2019) CAR-T cell therapy: a potential new strategy against prostate cancer[J]. J Immunother Cancer 7(1):258
Sena LA, Denmeade SR, Antonarakis ES (2021) Targeting the spectrum of immune checkpoints in prostate cancer[J]. Expert Rev Clin Pharmacol 14(10):1253–1266
Bian Z, Fan R, Xie L (2022) A novel cuproptosis-related prognostic gene signature and validation of differential expression in clear cell renal cell carcinoma. Genes (Basel) 13(5)
Davis CI, Gu X, Kiefer RM et al (2020) Altered copper homeostasis underlies sensitivity of hepatocellular carcinoma to copper chelation[J]. Metallomics 12(12):1995–2008
Scagliola A, Mainini F, Cardaci S (2020) The tricarboxylic acid cycle at the crossroad between cancer and immunity[J]. Antioxid Redox Signal 32(12):834–852
Vander HM, DeBerardinis RJ (2017) Understanding the intersections between metabolism and cancer biology[J]. Cell 168(4):657–669
Jiang P, Gu S, Pan D et al (2018) Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response[J]. Nat Med 24(10):1550–1558
Cheng X, Zeng Z, Yang H et al (2023) Novel cuproptosis-related long non-coding RNA signature to predict prognosis in prostate carcinoma[J]. BMC Cancer 23(1):105
Jiang S, Li Z, Dou R et al (2022) Construction and validation of a novel cuproptosis-related long noncoding RNA signature for predicting the outcome of prostate cancer[J]. Front Genet 13:976850
Jin L, Mei W, Liu X et al (2022) Identification of cuproptosis-related subtypes, the development of a prognosis model, and characterization of tumor microenvironment infiltration in prostate cancer[J]. Front Immunol 13:974034
Lv H, Liu X, Zeng X et al (2022) Comprehensive analysis of cuproptosis-related genes in immune infiltration and prognosis in melanoma[J]. Front Pharmacol 13:930041
Zhang Z, Zeng X, Wu Y et al (2022) Cuproptosis-related risk score predicts prognosis and characterizes the tumor microenvironment in hepatocellular carcinoma[J]. Front Immunol 13:925618
Wang W, Lu Z, Wang M et al (2022) The cuproptosis-related signature associated with the tumor environment and prognosis of patients with glioma[J]. Front Immunol 13:998236
Funding
This work was supported by Hebei Medical Science Research Program, No: 20221695.
Author information
Authors and Affiliations
Contributions
FY designed and conducted the study. HC, YC, and SL analyzed the data. CL, FY, and YX wrote and revised the manuscript. FY had read and approved the manuscript before its submission.
Corresponding author
Ethics declarations
Ethical Approval
All data in this study are open source and do not require ethics committee review.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Xiao, Y., Cao, H. et al. Cuproptosis Regulates Microenvironment and Affects Prognosis in Prostate Cancer. Biol Trace Elem Res 202, 99–110 (2024). https://doi.org/10.1007/s12011-023-03668-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03668-2