Abstract
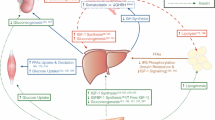
Increasing evidence suggests that organic vanadium compounds are bioavailable and safe therapeutic agents with insulin-mimetic and insulin-enhancing features. The objective of the current study was to examine the effect of vanadium-enriched yeast (VEY) supplementation on the gene expression level of insulin receptor substrates and clinical manifestations of obese type 2 diabetic mellitus (T2DM) patients. In this randomized, double-blind, placebo-controlled clinical trial, 44 obese T2DM patients were randomly allocated into either VEY (0.9 mg/day vanadium pentoxide) or placebo group for 12 weeks. The mRNA expression level of protein tyrosine phosphatase 1B (PTP1B), phosphatase and tensin homolog (PTEN), mitogen-activated protein kinase (MAPK), ribosomal protein S6 kinase (S6K), and nuclear factor kappa-light-chain-enhancer of activated B cells (NFƘB) genes in the peripheral blood mononuclear cells, serum levels of metabolic parameters, anthropometric indices, as well as the quality of life, and dietary intake were collected at pre- and post-intervention phases. Analysis of covariance was performed to obtain the corresponding effect size. Results showed that VEY administration significantly decreased anthropometric indices and glycemic parameters and increased insulin sensitivity after adjusting for potential covariates (p < 0.05), in comparison to the placebo group. Additionally, VEY supplementation was significantly effective on MAPK, PTP1B, and NFƘB gene expression level, compared to the placebo group. No significant changes were noticed for dietary intake, quality of life, and lipid profile in the VEY group, compared to the placebo group. Overall, VEY supplementation can be considered as a promising safe adjunct therapy for improving anthropometric indices and glycemic parameters in T2DM patients.



Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included within the article.
References
Alberti KGMM, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Med 15(7):539–53. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7%3c539::AID-DIA668%3e3.0.CO;2-S
International Diabetes Federation (2021) IDF Diabetes Atlas, 10th edn. Brussels, Belgium
Davies MJ, Aroda V, Collins B et al (2022) Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45(11):2753–2786. https://doi.org/10.2337/dci22-00344
Król E, Krejpcio Z, Michalak S, Wójciak RW, Bogdański P (2012) Effects of combined dietary chromium (III) propionate complex and thiamine supplementation on insulin sensitivity, blood biochemical indices, and mineral levels in high-fructose-fed rats. Biol Trace Elem Res 150(1):350–359. https://doi.org/10.1007/s12011-012-9515-5
Mishra S, Kumar A, Chaturvedi RK, Pandeya SN (2012) Vanadium salts versus diabetes: an overview. Sys Rev Pharm 1(2):172–174. https://doi.org/10.4103/0975-8453.75073
Treviño S, Diaz A (2020) Vanadium and insulin: partners in metabolic regulation. J Inorg Biochem 208:111094. https://doi.org/10.1016/j.jinorgbio.2020.111094
Liu J, Bao W, Jiang M, Zhang Y, Zhang X, Liu L (2012) Chromium, selenium, and zinc multimineral enriched yeast supplementation ameliorates diabetes symptom in streptozocin-induced mice. Biol Trace Elem Res 146(2):236–245. https://doi.org/10.1007/s12011-011-9248-x
Thompson KH, Liboiron BD, Sun Y, Bellman KD, Setyawati IA, Patrick BO et al (2003) Preparation and characterization of vanadyl complexes with bidentate maltol-type ligands; in vivo comparisons of anti-diabetic therapeutic potential. JBIC 8(1):66–74. https://doi.org/10.1007/s00775-002-0388-5
Thompson KH, Lichter J, LeBel C, Scaife MC, McNeill JH, Orvig C (2009) Vanadium treatment of type 2 diabetes: a view to the future. J Inorg Biochem 103(4):554–558. https://doi.org/10.1016/j.jinorgbio.2008.12.003
Thompson KHOC (2006) Vanadium in diabetes: 100 years from phase 0 to phase I. J Inorg Biochem 100:1925–35. https://doi.org/10.1016/j.jinorgbio.2006.08.016
Goc A (2006) Biological activity of vanadium compounds. Cent Eur J Biol 1(3):314–332. https://doi.org/10.2478/s11535-006-0029-z
Salice VCC, DummCL G (1999) Etcheverry SB tyrosine phosphorylation and morphological transformation induced by four vanadium compounds on MC3T3E1 cells. Mol Cell Biochem 198:119–128. https://doi.org/10.1023/A:1006997830346
Goldfine AB, Simonson DC, Folli F, Patti M-E, Kahn CR (1995) Metabolic effects of sodium metavanadate in humans with insulin-dependent and noninsulin-dependent diabetes mellitus in vivo and in vitro studies. J Clin Endocrinol Metab 80(11):3311–3320. https://doi.org/10.1210/jcem.80.11.7593444
Hei Y-j, Chen X, Pelech SL, Diamond J, McNeill JH (1995) Skeletal muscle mitogen-activated protein kinases and ribosomal S6 kinases: suppression in chronic diabetic rats and reversal by vanadium. Diabetes 44(10):1147–1155. https://doi.org/10.2337/diab.44.10.1147
Yong-jiang Hei SF, Chen X, Mary L, BaJH McNeill (1998) Stimulation of MAP kinase and S6 kinase by vanadium and selenium in rat adipocytes. Mol Cell Biochem 178:367–375. https://doi.org/10.1023/a:1006819906820
Semiz S (2022) Vanadium as potential therapeutic agent for COVID-19: a focus on its antiviral, antiinflamatory, and antihyperglycemic effects. J Trace Elem Med Biol 69:126887. https://doi.org/10.1016/j.jtemb.2021.126887
Moreira LPD, Gomes JVP, Mattar JB et al (2019) Potential of trace elements as supplements for the metabolic control of type 2 diabetes mellitus: a systematic review. JFF 57:317–327. https://doi.org/10.1016/j.jff.2019.04.015
Domingo JL, Gómez M (2016) Vanadium compounds for the treatment of human diabetes mellitus: a scientific curiosity? A review of thirty years of research. FCT 95:137–141. https://doi.org/10.1016/j.fct.2016.07.005
Ghalichi F, Ostadrahimi A, Saghafi-Asl M (2022) Vanadium and diabetic dyslipidemia: a systematic review of animal studies. J Trace Elem Med Biol 71:1–20. https://doi.org/10.1016/j.jtemb.2022.126955
Ghalichi F, Ostadrahimi A, Saghafi-Asl M (2022) Vanadium and biomarkers of inflammation and oxidative stress in diabetes: a systematic review of animal studies. HPP 12(2):122–130. https://doi.org/10.34172/hpp.2022.16
Habtewolde (2012) Effects of vanadium compounds on glycemic control in type 2 diabetes mellitus: a meta-analysis of comparative study on rats. IJPSR 3(10):3717–3724. https://doi.org/10.13040/IJPSR.0975-8232.3(10).3717-24
Nahas R, Moher M (2009) Complementary and alternative medicine for the treatment of type 2 diabetes. CFP 55(6):591–596
Smith DMP, Lewith GT (2008) A systematic review of vanadium oral supplements for glycaemic control in type 2 diabetes mellitus. QJM 101:351–358. https://doi.org/10.1093/qjmed/hcn003
Yeh G, Eisenberg D, Kaptchuk T et al (2003) Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 26(4):1277–1294. https://doi.org/10.2337/diacare.26.4.1277
Ulbricht C, Chao W, Costa D et al (2012) An evidence-based systematic review of vanadium by the natural standard research collaboration. J Diet Suppl 9(3):223–251. https://doi.org/10.3109/19390211.2012.709365
Afkhami-Arekani M, Karimi M, Mohammadi Mohammad S, Nourani F (2008) Effect of sodium metavanadate supplementation on lipid and glucose metabolism biomarkers in type e diabetic patients. Malays J Nutr 14(1):113–119
Boden G, Chen X, Ruiz J, van Rossum GD, Turco S (1996) Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non—insulin-dependent diabetes mellitus. Metabolism 45(9):1130–1135. https://doi.org/10.1016/s0026-0495(96)90013-x
Cohen N, Halberstam M, Shlimovich P, Chang CJ, Shamoon H, Rossetti L (1995) Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J Clin Investig 95(6):2501–2509. https://doi.org/10.1172/JCI117951
Cusi K, Cukier S, DeFronzo R, Torres M, Puchulu F, Redondo JP (2001) Vanadyl sulfate improves hepatic and muscle insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab 86(3):1410–1417. https://doi.org/10.1210/jcem.86.3.7337
Hamwi GJ (1964) Therapy: changing dietary concepts. Diabetes Mellitus: diagnosis and treatment 1:73–78
Soleimanzadeh H, Niaei A, Salari D, Tarjomannejad A, Penner S, Grünbacher M et al (2019) Modeling and optimization of V2O5/TiO2 nanocatalysts for NH3-selective catalytic reduction (SCR) of NOx by RSM and ANN techniques. J Environ Manage 238:360–367. https://doi.org/10.1016/j.jenvman.2019.03.018
Esmaeili S, Khosravi-Darani K, Pourahmad R, Nazemi L, Komeili R (2012) Production of selenium-enriched yeast using a Plackett-Burman design. Iran. J Nutr Sci 7(2):27–36. http://nsft.sbmu.ac.ir/article-1-767-en.html
Zare H, Owlia P, Vahidi H, Khujin MH (2018) Simultaneous optimization of the production of organic selenium and cell biomass in Saccharomyces cerevisiae by Plackett-Burman and Box-Behnken Design. IJPR 17(3):1081. http://research.shahed.ac.ir/WSR/WebPages/Report/PaperView.aspx?PaperID=85741
Pugazhenthi S, Khandelwal RL (1990) Insulinlike effects of vanadate on hepatic glycogen metabolism in nondiabetic and streptozocin-induced diabetic rats. Diabetes 39(7):821–827. https://doi.org/10.2337/diab.39.7.821
Burroughs TE, Desikan R, Waterman BM, Gilin D, McGill J (2004) Development and validation of the diabetes quality of life brief clinical inventory. Diabetes spectrum 17(1):41–49. https://doi.org/10.2337/diaspect.17.1.41
Rzewnicki R, Auweele YV, De Bourdeaudhuij I (2003) Addressing overreporting on the International Physical Activity Questionnaire (IPAQ) telephone survey with a population sample. Public Health Nutr 6(3):299–305. https://doi.org/10.1079/PHN2002427
Majid H, Masood Q, Khan AH (2017) Homeostatic model assessment for insulin resistance (HOMA-IR): a better marker for evaluating insulin resistance than fasting insulin in women with polycystic ovarian syndrome. J Coll Physicians Surg Pak 27(3):123–126
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85(7):2402–2410. https://doi.org/10.1210/jcem.85.7.6661
Karkhaneh A, Bagherieh M, Sadeghi S, Kheirollahi A (2019) Evaluation of eight formulas for LDL-C estimation in Iranian subjects with different metabolic health statuses. Lipids Health Dis 18(1):1–11. https://doi.org/10.1186/s12944-019-1178-1
Mishra P, Pandey CM, Singh U, Gupta A, Sahu C, Keshri A (2019) Descriptive statistics and normality tests for statistical data. Ann Card Anaesth 22(1):67. https://doi.org/10.4103/aca.ACA_157_18
Halberstam M, Cohen N, Shlimovich P, Rossetti L, Shamoon H (1996) Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects. Diabetes 45(5):659–666. https://doi.org/10.2337/diab.45.5.659
Wu Y, Huang M, Zhao P, Yang X (2013) Vanadyl acetylacetonate upregulates PPARγ and adiponectin expression in differentiated rat adipocytes. JBIC 18(6):623–631. https://doi.org/10.1007/s00775-013-1007-3
Russell R, Beard JL, Cousins RJ et al (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. A report of the panel on micronutrients, subcommittees on upper reference levels of nutrients and of interpretation and uses of dietary reference intakes, and the standing committee on the scientific evaluation of dietary reference intakes. Food and Nutrition Board, Institute of Medicine. Washington, DC: National Academy Press
Mohamed E, Mohamed M, Rashid FA (2004) Dyslipidaemic pattern of patients with type 2 diabetes mellitus. MJMS 11(1):44–51
Jacques-Camarena O, González-Ortiz M, Martínez-Abundis E, López-Madrueño JP, Medina-Santillán R (2008) Effect of vanadium on insulin sensitivity in patients with impaired glucose tolerance. Ann Nutr Metab 53(3–4):195–198. https://doi.org/10.1159/000175844
Mohammad A, Wang J, McNeill JH (2002) Bis(maltolato)oxovanadium(IV) inhibits the activity of PTP1B in Zucker rat skeletal muscle in vivo. Mol Cell Biochem 229(1):125–128. https://doi.org/10.1023/a:1017984930836
McNeill SSaJH (2002) Oral treatment with vanadium of Zucker fatty rats activates muscle glycogen synthesis and insulin-stimulated protein phosphatase-1 activity. Mol Cell Biochem 236:123–31. https://doi.org/10.1023/A:1016116700632
Wei D, Li M, Ding W (2007) Effect of vanadate on gene expression of the insulin signaling pathway in skeletal muscle of streptozotocin-induced diabetic rats. JBIC 12(8):1265–1273. https://doi.org/10.1007/s00775-007-0294-y
Stoker EIaAW (2017) Vanadium compounds as PTP inhibitors. Molecules 22(2269):5–19. https://doi.org/10.3390/molecules22122269
Mao L-L, Hao D-L, Mao X-W, Xu Y-F, Huang T-T, Wu B-N, Wang L-H (2015) Neuroprotective effects of bisperoxovanadium on cerebral ischemia by inflammation inhibition. Neurosci Lett 602:120–5. https://doi.org/10.1016/j.neulet.2015.06.040
Acknowledgements
The authors are grateful for the patient’s participation in this study. Our gratitude also goes to the vice chancellor for research (VCR) of Tabriz University of Medical Sciences (TBZMED) for their financial support. This study is based on the data obtained from a Ph.D. dissertation (Grant number: 67355) submitted to Tabriz University of Medical Sciences (Faezeh Ghalichi).
Author information
Authors and Affiliations
Contributions
FGH, AO, and MSA designed the research and contributed to the concept of the project, the development of the overall research plan, and study oversight. FGH drafted the manuscript, analyzed, and interpreted the data. AHF helped with analyzing the data and editing the manuscript. BK took part in the production of VEY capsules. AAN and MRJ were involved in the sampling and data collection. MSA edited the whole manuscript. All authors have given final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghalichi, F., Saghafi-Asl, M., Kafil, B. et al. Insulin Receptor Substrates Regulation and Clinical Responses Following Vanadium-Enriched Yeast Supplementation in Obese Type 2 Diabetic Patients: a Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Biol Trace Elem Res 201, 5169–5182 (2023). https://doi.org/10.1007/s12011-023-03604-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03604-4