Abstract
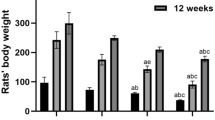
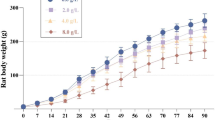
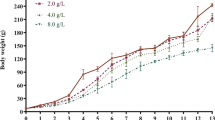
Aluminum has been found to be closely related to the pathogenesis of neurodegenerative diseases and damage learning and memory functions. Many changes in epigenetics may be one of the mechanisms of aluminum neurotoxicity. The purpose of this study is to further investigate the mechanism of action of sub-chronic aluminum exposure on learning memory and histone H4 acetylation modification in Wistar rats, and the correlation between learning memory impairment and histone H4 acetylation in aluminum-exposed rats. Rats in each dose group were given 0.0 g/L, 2.0 g/L, 4.0 g/L, and 8.0 g/L of AlCl3 distilled water daily for 12 weeks. The learning and memory ability of rats was measured by the Morris water maze test; the neuronal morphology of rat hippocampus was observed by Nissl staining and transmission electron microscope; real-time PCR, and Western blot were used to detect mRNA expression and protein content in hippocampus of rats. The results suggest that aluminum may affect the gene and protein expression of HAT1 and HDAC2, and then affect histone H4 and the acetylation of H4K12 (acH4K12), which may lead to learning and memory dysfunction in rats.










Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Yousef MI, Roychoudhury S, Jafaar KS, Slama P, Kesari KK, Kamel MA (2022) Aluminum oxide and zinc oxide induced nanotoxicity in rat brain, heart, and lung. Physiol Res. 71(5):677–694. https://doi.org/10.33549/physiolres.934831
Walton JR (2009) Functional impairment in aged rats chronically exposed to human range dietary aluminum equivalents. Neurotoxicology 30(2):182–193. https://doi.org/10.1016/j.neuro.2008.11.012
Platt B, Fiddler G, Riedel G et al (2001) Aluminium toxicity in the rat brain: histochemical and immunocytochemical evidence. Brain Res Bull 55(2):257–267. https://doi.org/10.1016/S0361-9230(01)00511-1
Niu Q (2018) Overview of the relationship between aluminum exposure and health of human being. Adv Exp Med Biol. 1091:1–31. https://doi.org/10.1007/978-981-13-1370-7_1
Sun W, Li J, Li X et al (2022) Aluminium oxide nanoparticles compromise spatial memory performance and proBDNF-mediated neuronal function in the hippocampus of rats. 19(1):34. Published 2022 May 10. https://doi.org/10.1186/s12989-022-00477-8
Abbas F, Eladl MA, El-Sherbiny M et al (2022) Celastrol and thymoquinone alleviate aluminum chloride- induced neurotoxicity: Behavioral psychomotor performance, neurotransmitter level, oxidative -inflammatory markers, and BDNF expression in rat brain. Biomed Pharmacother. 151:113072. https://doi.org/10.1016/j.biopha.2022.113072
Nie J (2018) Exposure to aluminum in daily life and alzheimer's disease. Adv Exp Med Biol. 1091:99–111. https://doi.org/10.1007/978-981-13-1370-7_6
Zhang L, Jin C, Liu Q et al (2013) Effects of subchronic aluminum exposure on spatial memory, ultrastructure and L-LTP of hippocampus in rats. J Toxicol Sci 38(2):255–268. https://doi.org/10.2131/jts.38.255
Zhang L, Jin C, Lu X et al (2014) Aluminium chloride impairs long-term memory and downregulates cAMP-PKA-CREB signalling in rats. Toxicology 323:95–108. https://doi.org/10.1016/j.tox.2014.06.011
Li H, Xue X, Li Z et al (2020) Aluminium-induced synaptic plasticity injury via the PHF8- H3K9me2-BDNF signalling pathway. Chemosphere. 244:125445. https://doi.org/10.1016/j.chemosphere.2019.125445
Wang F, Kang P, Li Z et al (2019) Role of MLL in the modification of H3K4me3 in aluminium- induced cognitive dysfunction. Chemosphere 232:121–129. https://doi.org/10.1016/j.chemosphere.2019.05.099
Wu Y, Wang R, Liu R, Ba Y, Huang H (2023) The roles of histone modifications in metal-induced neurological disorders. Biol Trace Elem Res 201(1):31–40. https://doi.org/10.1007/s12011-022-03134-5
van Holde K, Zlatanova J (2007) Chromatin fiber structure: Where is the problem now? Semin Cell Dev Biol. 18(5):651–658. https://doi.org/10.1016/j.semcdb.2007.08.005
Conrad T, Akhtar A (2012) Dosage compensation in Drosophila melanogaster: epigenetic fine-tuning of chromosome-wide transcription. Nat Rev Genet 13(2):123–134. https://doi.org/10.1038/nrg3124
Saha RN, Pahan K (2006) HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ 13(4):539–550. https://doi.org/10.1038/sj.cdd.4401769
Xu H, Ye M, Xia A et al (2022) The Fng3 ING protein regulates H3 acetylation and H4 deacetylation by interacting with two distinct histone-modifying complexes. New Phytol. 235(6):2350–2364. https://doi.org/10.1111/nph.18294
Meyer-Baron M, Schäper M, Knapp G et al (2007) Occupational aluminum exposure: evidence in support of its neurobehavioral impact. Neurotoxicology 28(6):1068–1078. https://doi.org/10.1016/j.neuro.2007.07.001
Campbell A (2002) The potential role of aluminium in Alzheimer's disease. Nephrol Dial Transplant 17(Suppl 2):17–20. https://doi.org/10.1093/ndt/17.suppl_2.17
Gupta VB, Anitha S et al (2005) Aluminium in Alzheimer's disease: are we still at a crossroad? Cell Mol Life Sci 62(2):143–158. https://doi.org/10.1007/s00018-004-4317-3
Nativio R, Donahue G et al (2018) Dysregulation of the epigenetic landscape of normal aging in Alzheimer's disease. Nat Neurosci 21(4):497–505. https://doi.org/10.1038/s41593-018-0101-9
Shang A, Bieszczad KM (2022) Epigenetic mechanisms regulate cue memory underlying discriminative behavior. Neurosci Biobehav Rev. 141:104811. https://doi.org/10.1016/j.neubiorev.2022.104811
Henry KW, Wyce A et al (2003) Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev 17(21):2648–2663. https://doi.org/10.1101/gad.1144003
Kitahara M, Inoue T, Mani H et al (2021) Exercise and pharmacological inhibition of histone deacetylase improves cognitive function accompanied by an increase of gene expressions crucial for neuronal plasticity in the hippocampus. Neurosci Lett 749:135749. https://doi.org/10.1016/j.neulet.2021.135749
Wang S, Zhang X, Wang Q, Wang R (2022) Histone modification in podocyte injury of diabetic nephropa- thy. J Mol Med (Berl). 100(10):1373–1386. https://doi.org/10.1007/s00109-022-02247-7
Ramazi S, Allahverdi A, Zahiri J (2020) Evaluation of post-translational modifications in histone proteins: A review on histone modification defects in developmental and neurological disorders. J Biosci. 45:135
Gao S, Li L, Han X et al (2021) Genome-wide identification of the histone acetyltransferase gene family in Triticum aestivum. BMC Genomics 22(1):49. Published 2021 Jan 11. https://doi.org/10.1186/s12864-020-07348-6
Barnes CE, English DM, Cowley SM (2019) Acetylation & Co: an expanding repertoire of histone acylations regulates chromatin and transcription. Essays Biochem 63(1):97–107. https://doi.org/10.1042/ebc20180061
Agalioti T, Chen G, Thanos D (2002) Deciphering the transcriptional histone acetylation code for a human gene. Cell 111(3):381–392. https://doi.org/10.1016/s0092-8674(02)01077-2
Schübeler D, MacAlpine DM, Scalzo D et al (2004) The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev 18(11):1263–1271. https://doi.org/10.1101/gad.1198204
Poziello A, Nebbioso A, Stunnenberg HG, Martens JHA, Carafa V, Altucci L (2021) Recent insights into Histone Acetyltransferase-1: biological function and involvement in pathogenesis. Epigenetics. 16(8):838–850. https://doi.org/10.1080/15592294.2020.1827723
Peleg S, Sananbenesi F, Zovoilis A et al (2010) Altered histone acetylation is associated with age- dependent memory impairment in mice. Science 328(5979):753–756. https://doi.org/10.1126/science.1186088
Ito K, Yamamura S, Essilfie-Quaye S et al (2006) Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 203(1):7–13. https://doi.org/10.1084/jem.20050466
Wu J, Dong L, Zhang M et al (2013) Class I histone deacetylase inhibitor valproic acid reverses cognitive deficits in a mouse model of septic encephalopathy. Neurochem Res 38(11):2440–2449. https://doi.org/10.1007/s11064-013-1159-0
Nieto-Estevez V, Changarathil G, Adeyeye AO et al (2022) HDAC1 regulates neuronal differentiation. Front Mol Neurosci 14:815808. Published 2022 Jan 12. https://doi.org/10.3389/fnmol.2021.815808
Vandebroek A, Yasui M (2020) Regulation of AQP4 in the Central Nervous System. Int J Mol Sci. 21(5):1603. https://doi.org/10.3390/ijms21051603
Drummond DC, Noble CO et al (2005) Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol 45:495–528. https://doi.org/10.1146/annurev.pharmtox.45.120403.095825
Fraga MF, Ballestar E et al (2005) Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37(4):391–400. https://doi.org/10.1038/ng1531
Ren C, Zhang L, Freitas MA et al (2005) Peptide mass mapping of acetylated isoforms of histone H4 from mouse lymphosarcoma cells treated with histone deacetylase (HDACs) inhibitors. J Am Soc Mass Spectrom 16(10):1641–1653. https://doi.org/10.1016/j.jasms.2005.06.001
Su X, Zhang L, Lucas DM et al (2007) Histone H4 acetylation dynamics determined by stable isotope labeling with amino acids in cell culture and mass spectrometry. Anal Biochem 363(1):22–34. https://doi.org/10.1016/j.ab.2006.12.031
Zhang L, Su X, Liu S et al (2007) Histone H4 N-terminal acetylation in Kasumi-1 cells treated with depsipeptide determined by acetic acid-urea polyacrylamide gel electrophoresis, amino acid coded mass tagging, and mass spectrometry. J Proteome Res 6(1):81–88. https://doi.org/10.1021/pr060139u
Li Y, Seto E (2016) HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med. 6(10):a026831. https://doi.org/10.1101/cshperspect.a026831
Acknowledgements
The authors sincerely appreciate the support of everyone. Thank the correspondent for guiding the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (81673226); by the Initiated Research Foundation for the Doctoral Program of Science and Technology Department of Liaoning Province, China (201601226); by the Natural Science Foundation of Education Department of Liaoning Province, China (L2015544, LJKZ1146); by the Natural Science Foundation for Innovation and Entrepreneurship Training Program of Education Department of Liaoning Province, China (201710164000038); by the Natural Science Foundation of Science and Technology Department of Shenyang City, China (17–231-1–44); by the Natural Science Foundation of Shenyang Medical College, China (20153043); by the Natural Science Foundation for graduate students of Shenyang Medical College, China (Y20180512); and by the Natural Science Foundation for undergraduate students of Shenyang Medical College, China (20179028).
Author information
Authors and Affiliations
Contributions
Jie Gao: conceptualization, methodology, software, data curation, writing—original draft, writing–review and editing. Shiming Zhang: methodology, data curation, writing—original draft. Bing Li: methodology, data curation, writing—original draft. Ziyi Wang: methodology, data curation, writing—original draft. Wei Liu: methodology, data curation, writing—original draft. Lifeng Zhang: conceptualization, methodology, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Disclosure Statement
The authors acknowledge that they have read the Journal's position on ethical publishing issues and certify that this manuscript conforms to the Journal's guidelines.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, J., Zhang, S., Li, B. et al. Sub-Chronic Aluminum Exposure in Rats’ Learning-Memory Capability and Hippocampal Histone H4 Acetylation Modification: Effects and Mechanisms. Biol Trace Elem Res 201, 5309–5320 (2023). https://doi.org/10.1007/s12011-023-03602-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03602-6