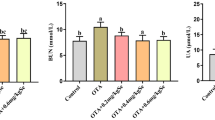
Abstract
The single and combined effects of short-term selenium (Se) deficiency and T-2 toxin-induced kidney pathological injury through the MMPs/TIMPs system were investigated. Forty-eight rats were randomly divided into control, 10 ng/g T-2 toxin, 100 ng/g T-2 toxin, Se-deficient, 10 ng/g T-2 toxin and Se deficiency combined, and 100 ng/g T-2 toxin and Se deficiency combined groups for a 4-week intervention. The kidney Se concentration was measured to evaluate the construction of animal models of Se deficiency. Kidney tissues were analyzed by hematoxylin–eosin staining, Masson staining, and transmission electron microscope to observe the pathological changes, the severity of kidney fibrosis, and ultrastructural changes, respectively. Meanwhile, quantitative polymerase chain reaction and immunohistochemical staining were used to analyze the gene and protein expression levels of matrix metallopeptidase 2/3 (MMP2/3) and tissue inhibitor of metalloproteinase 1 (TIMP1). The results showed that short-term Se deficiency and T-2 toxin exposure can cause kidney injury through tubular degeneration and even lead to kidney fibrosis. And the combination of T-2 toxin and Se deficiency had a synergistic effect on the kidney. A dose–response effect of the T-2 toxin was also observed. At the gene and protein levels, the expression of MMP2/3 in the intervention group increased, while the expression of TIMP1 decreased compared with the control group. In conclusion, short-term Se deficiency and T-2 toxin exposure might lead to injury and even the development of fibrosis in the kidneys, and combined intervention can increase the severity with a dose-dependent trend. MMP2/3 and TIMP1 likely play a significant role in the development of kidney fibrosis.






Similar content being viewed by others
References
Zhang LDY, Chen YZ, Xu JY, Wang B, Qin LQ (2020) New progress of selenium and hepatic injury. Modern Preventive Medicine 47(22):4218–4220
Kvícala J (1999) Selenium and the organism. Cas Lek Cesk 138(4):99–106
Rayman MP (2000) The importance of selenium to human health. The Lancet 356(9225):233–241
Qiao L, Lin X, Zhao Y, Wang Q, Liu H, You M, Yuan Q, Yang Z, Bian W, Liu J, Guo Z and Han J (2022) Shortterm dietary selenium deficiency induced liver fibrosis by inhibiting the Akt/mTOR signaling pathway in rats. Biol Trace Elem Res
Tan LC, Nancharaiah YV, van Hullebusch ED, Lens PNL (2016) Selenium: environmental significance, pollution, and biological treatment technologies. Biotechnol Adv 34(5):886–907
Fang W, Wu P, Hu R, Huang Z (2003) Environmental Se–Mo–B deficiency and its possible effects on crops and Keshan-Beck disease (KBD) in the Chousang Area, Yao County, Shaanxi Province. Chin Environ Geochem Health 25(2):267–280
Yan C, Luo R, Li F, Liu M, Li J, Hua W, Li X (2021) The epidemiological status, environmental and genetic factors in the etiology of Keshan disease. Cardiovasc Endocrinol Metab 10(1):14–21
Qiao L, Guo Z, Liu H, Liu J, Lin X, Deng H, Liu X, Zhao Y, Xiao X, Lei J, Han J (2022) Protective effect of mitophagy regulated by mTOR signaling pathway in liver fibrosis associated with selenium. Nutrients 14(12):2410
Lai H, Nie T, Zhang Y, Chen Y, Tao J, Lin T, Ge T, Li F, Li H (2021) Selenium deficiency-induced damage and altered expression of mitochondrial biogenesis markers in the kidneys of mice. Biol Trace Elem Res 199(1):185–196
Han J, Liang H, Yi J, Tan W, He S, Wu X, Shi X, Ma J, Guo X (2016) Selenium deficiency induced damages and altered expressions of metalloproteinases and their inhibitors (MMP1/3, TIMP1/3) in the kidneys of growing rats. J Trace Elem Med Biol 34:1–9
Pu G, Liu A, Huang D, Wu Q, Yuan Z (2018) Brain damage and neurological symptoms induced by T-2 toxin in rat brain. Toxicol Lett 286:96–107
Yang X, Liu P, Cui Y, Xiao B, Li Y (2020) Review of the reproductive toxicity of T-2 toxin. J Agric Food Chem 68(3):727–734
Liu Y, Dong R, Yang Y, Xie H, Zhang Z (2021) Protective effect of organic selenium on oxidative damage and inflammatory reaction of rabbit kidney induced by T-2 toxin. Biol Trace Elem Res 199(5):1833–1842
Zhang X, Wang Y, Yang X, Liu M, Huang W, Zhang J, Song M, Shao B, Li Y (2021) The nephrotoxicity of T-2 toxin in mice caused by oxidative stress-mediated apoptosis is related to Nrf2 pathway. Food Chem Toxicol 149:112027
Rahman S, Sharma AK, Singh ND, Prawez S (2016) T-2 toxin induced nephrotoxicity in Wistar rats. Indian Journal of Veterinary Pathology 40(4):320
Xu J, Pan S, Gan F, Hao S, Liu D, Xu H, Huang K (2018) Selenium deficiency aggravates T-2 toxin-induced injury of primary neonatal rat cardiomyocytes through ER stress. Chem Biol Interact 285:96–105
Deng H, Chilufya MM, Liu J, Qiao L, Xiao X, Zhao Y, Guo Z, Lv Y, Wang W, Zhang J, Han J (2021) Effect of low nutrition and T-2 toxin on C28/I2 chondrocytes cell line and chondroitin sulfate-modifying sulfotransferases. Cartilage 13(2):818S-825S
Yao YF, Kang PD, Li XB, Yang J, Shen B, Zhou ZK, Pei FX (2010) Study on the effect of T-2 toxin combined with low nutrition diet on rat epiphyseal plate growth and development. Int Orthop 34(8):1351–1356
Rao VH, Lees GE, Kashtan CE, Nemori R, Singh RK, Meehan DT, Rodgers K, Berridge BR, Bhattacharya G, Cosgrove D (2003) Increased expression of MMP-2, MMP-9 (type IV collagenases/gelatinases), and MT1-MMP in canine X-linked Alport syndrome (XLAS). Kidney Int 63(5):1736–1748
Brew K, Nagase H (2010) The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803(1):55–71
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11):1939–1951
Fr S, Wang S, HS, HL, (2007) The state of mycotoxin maximum limit of grain. Science and Technology of Cereals, Oils and Foods 6:57–59
Zhang G, Chen Y, Bilalwaqar A, Han L, Jia M, Xu C, Yu Q (2015) Quantitative analysis of rabbit coronary atherosclerosis. Practical techniques utilizing open-source software. Anal Quant Cytopathol Histpathol 37(2):115–122
Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Bühler FR (1972) Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med 286(9):441–449
Seely JC, Hard GC, Blankenship B (2018) Kidney. In: Boorman’s Pathology of the Rat, pp 125–166
Janik E, Niemcewicz M, Podogrocki M, Ceremuga M, Stela M, Bijak M (2021) T-2 toxin-the most toxic trichothecene mycotoxin: metabolism, toxicity, and decontamination strategies. Molecules 26(22):6868
Korbut AI, Taskaeva IS, Bgatova NP, Muraleva NA, Orlov NB, Dashkin MV, Khotskina AS, Zavyalov EL, Konenkov VI, Klein T, Klimontov VV (2020) SGLT2 inhibitor empagliflozin and DPP4 inhibitor linagliptin reactivate glomerular autophagy in db/db mice, a model of type 2 diabetes. Int J Mol Sci 21(8):2987
Wiggins RC (2007) The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71(12):1205–1214
Patschan D, Plotkin M, Goligorsky MS (2006) Therapeutic use of stem and endothelial progenitor cells in acute renal injury: ca ira. Curr Opin Pharmacol 6(2):176–183
Garg P (2018) A review of podocyte biology. Am J Nephrol 47(Suppl 1):3–13
Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV (2013) The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol 304(4):F333–F347
Tyagi I, Agrawal U, Amitabh V, Jain AK, Saxena S (2008) Thickness of glomerular and tubular basement membranes in preclinical and clinical stages of diabetic nephropathy. Indian J Nephrol 18(2):64–69
Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noel LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA, Renal Pathology S (2010) Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21(4):556–563
Panizo S, Martinez-Arias L, Alonso-Montes C, Cannata P, Martin-Carro B, Fernandez-Martin JL, Naves-Diaz M, Carrillo-Lopez N, Cannata-Andia JB (2021) Fibrosis in chronic kidney disease: pathogenesis and consequences. Int J Mol Sci 22(1):408
Chen X, Mu P, Zhu L, Mao X, Chen S, Zhong H, Deng Y (2021) T-2 toxin induces oxidative stress at low doses via Atf3DeltaZip2a/2b-mediated ubiquitination and degradation of Nrf2. Int J Mol Sci 22(15):7936
Liu X, Wang Z, Wang X, Yan X, He Q, Liu S, Ye M, Li X, Yuan Z, Wu J, Yi J, Wen L, Li R (2021) Involvement of endoplasmic reticulum stress-activated PERK-eIF2alpha-ATF4 signaling pathway in T-2 toxin-induced apoptosis of porcine renal epithelial cells. Toxicol Appl Pharmacol 432:115753
Zhou X, Yang H, Guan F, Xue S, Song D, Chen J, Wang Z (2016) T-2 toxin alters the levels of collagen II and its regulatory enzymes MMPs/TIMP-1 in a low-selenium rat model of Kashin-Beck disease. Biol Trace Elem Res 169(2):237–246
Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69(3):562–573
Chen QK, Lee K, Radisky DC, Nelson CM (2013) Extracellular matrix proteins regulate epithelial-mesenchymal transition in mammary epithelial cells. Differentiation 86(3):126–132
Catania JM, Chen G, Parrish AR (2007) Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol 292(3):F905–F911
Acknowledgements
The authors thank all study participants.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81872567).
Author information
Authors and Affiliations
Contributions
Study design: Jing Han and Ziwei Guo. Animal experiments: Ziwei Guo, Mumba mulutula Chilufya, Huan Deng, and Lichun Qiao. Data collection: Jiaxin Liu, Xiang Xiao, and Yan Zhao. Statistical analysis: Xue Lin and Haobiao Liu. Figure preparation: Mumba mulutula Chilufya, Xue Lin, and Rongqi Xiang. Manuscript preparation: Ziwei Guo and Jing Han. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
The animal study protocol was approved by the Medical Animal Research Ethics Committee of Xi’an Jiaotong University (protocol code 2018.263 and 6 March 2018 of approval).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Z., Chilufya, M.m., Deng, H. et al. Single and Combined Effects of Short-Term Selenium Deficiency and T-2 Toxin-Induced Kidney Pathological Injury Through the MMPs/TIMPs System. Biol Trace Elem Res 201, 4850–4860 (2023). https://doi.org/10.1007/s12011-023-03566-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03566-7