Abstract
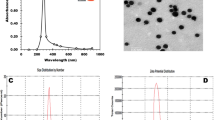
Different nanoparticles (NPs) are currently being investigated for their potential role as cryoprotectant during semen cryopreservation in several mammalian species. It may be possible to improve semen quality following cryopreservation by supplementation of NPs in the freezing extenders. The present study was carried out in semen collected from four (4) Assam Hill Goat bucks (10 ejaculates per buck) to investigate the effect of supplementing zinc oxide (ZnO) and selenium (Se) NPs in Tris-citric acid-fructose yolk (TCFY) extender on in vitro sperm quality and in vivo fertility rate after freeze-thawing. The size morphology and zeta potential of ZnO and Se NPs were evaluated prior to its incorporation in the freezing extender. Qualified semen samples (> 70% progressive motility) were divided into five (5) aliquots and then diluted in TCFY extender containing ZnO and Se NP supplementation at different concentrations (T0, control; T1, 0.1 mg/mL ZnO NPs; T2, 0.5 mg/mL ZnO NPs; T3, 0.5 µg/mL Se NPs; and T4, 1 µg/mL Se NPs). Diluted semen was packed in 0.25 mL straws and then stored in liquid nitrogen. After thawing, post-thaw in vitro sperm attributes were evaluated. Finally, the effect of NPs on in vivo fertility rate was checked in heat-synched does (n = 70) by artificial insemination (AI) using straws that showed superior results during the in vitro study. Results showed that ZnO and Se NPs were poly-crystalline in nature with particle size below 100 nm (nm). The evaluated post-thaw sperm in vitro attributes were significantly (p < 0.001) higher in T1 in comparison to T0. The antioxidant enzyme activities were significantly (p < 0.001) higher in T1. Lipid peroxidation (LPO) profile was significantly (p < 0.001) lower in T1. Sperm motility and mitochondrial membrane potential (MMP) had a highly significant (r = 0.580, p < 0.05) association in T1. No significant (p > 0.05) differences in pregnancy rates were recorded after AI in the different treatments. In conclusion, extender supplemented with 0.1 mg/mL ZnO NPs improved post-thaw semen quality of goat spermatozoa consequently by increasing activities of endogenous antioxidant enzymes thereby lowering LPO levels. However, improved in vitro outcomes might not correspond to improved field fertility outcomes.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Purdy PH (2006) A review on goat sperm cryopreservation. Small Rumin Res 63:215–225. https://doi.org/10.1016/j.smallrumres.2005.02.015
Collin S, Sirard MA, Dufour M, Bailey JL (2000) Sperm calcium levels and chlortetracycline fluorescence patterns are related to the in vivo fertility of cryopreserved bovine semen. J Androl 21:938–943. https://doi.org/10.1002/j.1939-4640.2000.tb03425.x
Bell M, Wang R, Hellstrom WJ, Sikka SC (1993) Effect of cryoprotective additives and cryopreservation protocol on sperm membrane lipid peroxidation and recovery of motile human sperm. J Androl 14:472–478. https://doi.org/10.1002/j.1939-4640.1993.tb03266.x
Bilodeau JF, Blanchette S, Gagnon IC, Sirard MA (2001) Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology 56:275–286. https://doi.org/10.1016/s0093-691x(01)00562-3
Storey BT (1997) Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod 3:203–213. https://doi.org/10.1093/molehr/3.3.203
Çoyan K, Başpınar N, Bucak MN et al (2010) Influence of methionine and dithioerythritol on sperm motility, lipid peroxidation and antioxidant capacities during liquid storage of ram semen. Res Vet Science 89:426–431. https://doi.org/10.1016/j.rvsc.2010.03.025
AminiPour H, Tahmasbi AM, Naserian AA (2013) The influence of vitamin E on semen characteristics of Ghezel rams in during cooling and frozen process. Eur J Zool Res 2:94–99
Maneesh M, Jayalekshmi H (2006) Role of reactive oxygen species and antioxidants on pathophysiology of male reproduction. Indian J Clin Biochem 21:80–89. https://doi.org/10.1007/BF02912918
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042. https://doi.org/10.1021/np9904509
Forouzanfar M, Sharafi M, Hosseini SM et al (2010) In vitro comparison of egg yolk–based and soybean lecithin–based extenders for cryopreservation of ram semen. Theriogenology 73:480–487. https://doi.org/10.1016/j.theriogenology.2009.10.005
Shahin MA, Khalil WA, Saadeldin IM, Swelum AA, El-Harairy MA (2021) Effects of vitamin C, vitamin E, selenium, zinc, or their nanoparticles on camel epididymal spermatozoa stored at 4° C. Trop Anim Health Prod 53:1–9. https://doi.org/10.1007/s11250-020-02521-1
Hashem NM, Gonzalez-Bulnes A (2020) State-of-the-art and prospective of nanotechnologies for smart reproductive management of farm animals. Animals 10:840. https://doi.org/10.3390/ani10050840
Eggert-Kruse W, Zwick EM, Batschulat K, Rohr G, Armbruster P, Petzoldt D, Strowitzki T (2002) Are zinc levels in seminal plasma associated with seminal leukocytes and other determinants of semen quality? Fertil Steril 77:260–269. https://doi.org/10.1016/s0015-0282(01)02974-0
Mayasula VK, Arunachalam A, Sellappan S, Guvvala PR, Ghosh J (2022) Organic zinc and copper supplementation–associated changes in gene expression and protein profiles in buck spermatozoa. Biol Trace Elem Res 200:1626–1639. https://doi.org/10.1007/s12011-021-02796-x
Afifi M, Abdelazim AM (2015) Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats. Asian Pac J Trop Biomed 5:874–877. https://doi.org/10.1016/j.apjtb.2015.06.010
Afifi M, Almaghrabi OA, Kadasa NM (2015) Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. BioMed Res Int. https://doi.org/10.1155/2015/153573
Talebi AR, Khorsandi L, Moridian M (2013) The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet 30:1203–1209. https://doi.org/10.1007/s10815-013-0078-y
Barkhordari A, Hekmatimoghaddam S, Jebali A, Khalili MA, Talebi A, Noorani M (2013) Effect of zinc oxide nanoparticles on viability of human spermatozoa. Iran J Reprod Med 11:767
Farhadi F, Towhidi A, Shakeri M, Seifi-Jamadi, (2022) A zincoxide nanoparticles have beneficial effect on frozen-thawed spermatozoa of Holstein bulls. Iran J Appl Anim Sci 12:49–55
Jahanbin R, Yazdanshenas P, Rahimi M, Hajarizadeh A, Tvrda E, Nazari SA, Mohammadi-Sangcheshmeh A, Ghanem N (2021) In vivo and in vitro evaluation of bull semen processed with zinc (zn) nanoparticles. Biol Trace Elem Res 99:126–135. https://doi.org/10.1007/s12011-020-02153-4
Pelyhe C, Mezes M (2013) Myths and facts about the effects of nano selenium in farm animals–mini-review. Eur Chem Bull 2:1049–1052. https://doi.org/10.17628/ECB.2013.2.1049-1052
Shi LG, Yang RJ, Yue WB, Xun WJ, Zhang CX, Ren YS, Shi L, Lei FL (2010) Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim Reprod Sci 118:248–254. https://doi.org/10.1016/j.anireprosci.2009.10.003
Talebi E, Ghasemi F, Haghigat Jahromi M (2014) Effect of selenium nanoparticles antioxidant on sperm parameters in mature and adult rats. J Fasa Univ Med Sci 4:111–119
Safa S, Moghaddam G, Jozani RJ, Kia HD, Janmohammadi H (2016) Effect of vitamin E and selenium nanoparticles on post-thaw variables and oxidative status of rooster semen. Anim Reprod Sci 174:100–106. https://doi.org/10.1016/j.anireprosci.2016.09.011
Kukov A, Daskalova D, Ivanova-Kicheva M, Stefanov R, Neicheva A (2009) Immunocytochemical localization of separated seminal plasma proteins on the ram’s sperm plasma membrane during in vitro preservation at 4ºC. Biotech Anim Husb 25:925–934
Lasley JF (1951) Spermatozoan motility as a measure of semen quality. J Anim Sci 10:211. https://doi.org/10.2527/jas1951.101211x
Jeyendran RS, Van Der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJD (1984) Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. Reproduction 70:219–228. https://doi.org/10.1530/jrf.0.0700219
Mendoza C, Carreras A, Moos J, Tesarik J (1992) Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil 95:755–763. https://doi.org/10.1530/jrf.0.0950755
Chohan KR, Griffin JT, Carrell DT (2004) Evaluation of chromatin integrity in human sperm using acridine orange staining with different fixatives and after cryopreservation. Andrologia 36:321–326. https://doi.org/10.1111/j.1439-0272.2004.00626.x
Selvaraju S, Ravindra JP, Ghosh J, Gupta PSP, Suresh KP (2008) Evaluation of sperm functional attributes in relation to in vitro sperm zona pellucida binding ability and cleavage rate in assessing frozen thawed buffalo (Bubalus bubalis) semen quality. Anim Reprod Sci 106:311–321. https://doi.org/10.1016/j.anireprosci.2007.05.005
Fraser LR, Abeydeera LR, Niwa K (1995) Ca2+-regulating mechanisms that modulate bull sperm capacitation and acrosomal exocytosis as determined by chlortetracycline analysis. Mol Reprod Dev 40:233–241. https://doi.org/10.1002/mrd.1080400213
Madesh M, Balasubramanian KA (1997) Activation of liver mitochondrial phospholipase A2 by superoxide. Arch Biochem Biophys 346:187–192. https://doi.org/10.1006/abbi.1997.0288
Bergmeyer HU (1974) Methods of enzymatic analysis. Elsevier, Weinheim
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Aitken RJ, Wingate JK, De Iuliis GN, McLaughlin EA (2007) Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol Hum Reprod 13:203–211. https://doi.org/10.1093/molehr/gal119
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxidase in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Holtz W, Sohnrey B, Gerland M, Driancourt MA (2008) Ovsynch synchronization and fixed-time insemination in goats. Theriogenology 69:785–792. https://doi.org/10.1016/j.theriogenology.2007.10.004
Xiong Y, Huang L, Mahmud S, Yang F, Liu H (2020) Bio-synthesized palladium nanoparticles using alginate for catalytic degradation of azo-dyes. Chin J Chem Eng 28:1334–1343. https://doi.org/10.1016/j.cjche.2020.02.014
Muino R, Tamargo C, Hidalgo CO, Pena AI (2008) Identification of sperm subpopulations with defined motility characteristics in ejaculates from Holstein bulls: effects of cryopreservation and between-bull variation. Anim Reprod Sci 109:27–39. https://doi.org/10.1016/j.anireprosci.2007.10.007
Halo M Jr, Bułka K, Antos PA, Gren A, Slanina T, Ondruškae L, Tokárová K, Massányi M, Formicki G, Halo M, Massanyi P (2021) The effect of ZnO nanoparticles on rabbit spermatozoa motility and viability parameters in vitro. Saudi J Biol Sci 28:7450–7454. https://doi.org/10.1016/j.sjbs.2021.08.045
Ahmed WM, El-Tohamy MM (1997) Zinc profile in blood and semen of breeding buffalo-bull with particular emphasis on age variation and semen characteristics. Proc 5th World Buffalo Congr R Palace Caserta 13–16 825–828.
Hozyen HF, El-Shamy AA (2019) Screening of genotoxicity and oxidative stress effect of selenium nanoparticles on ram spermatozoa. Curr Sci Int 7:799–807
Shahin MA, Khalil WA, Saadeldin IM, Swelum AA, El-Harairy MA (2020) Comparison between the effects of adding vitamins, trace elements, and nanoparticles to SHOTOR extender on the cryopreservation of dromedary camel epididymal spermatozoa. Animals 10:78. https://doi.org/10.3390/ani10010078
Khalil WA, El-Harairy MA, Zeidan AE, Hassan MA (2019) Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 126:121–127. https://doi.org/10.1016/j.theriogenology.2018.12.017
Barbosa FA, Souza GM (2006) Efeitos dos microminerais na reprodução de bovinos. Retrieved form www.agronomia.com.br/conteúdo/ artigos. Accessed 26 Jul 2022
Gualtieri R, Barbato V, Fiorentino I, Braun S, Rizos D, Longobardi S, Talevi R (2014) Treatment with zinc, d-aspartate, and coenzyme Q10 protects bull sperm against damage and improves their ability to support embryo development. Theriogenology 82:592–598. https://doi.org/10.1016/j.theriogenology.2014.05.028
Isaac AV, Kumari S, Nair R, Urs DR, Salian SR, Kalthur G, Adiga SK, Manikhath J, Mutalik S, Pasricha R (2017) Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem Biophys Res Commun 494:656–662. https://doi.org/10.1016/j.bbrc.2017.10.112
Nateq S, Moghaddam G, Alijani S, Behnam M (2020) The effects of different levels of nano selenium on the quality of frozen-thawed sperm in ram. J Appl Anim Res 48:434–439. https://doi.org/10.1080/09712119.2020.1816549
Vallorani C, Spinaci M, Bucci D, Tamanini C, Galeati G (2010) Effects of antioxidants on boar spermatozoa during sorting and storage. Anim Reprod Sc 122:58–65. https://doi.org/10.1016/j.anireprosci.2010.07.007
Michailov Y, Ickowicz D, Breitbart H (2014) Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev Biol 396:246–255. https://doi.org/10.1016/j.ydbio.2014.10.009
Hozyen HF, El-Shamy AA, Farghali AA (2019) In vitro supplementation of nano selenium minimizes freeze-thaw induced damage to ram spermatozoa. Int J Vet Sci 8:249–254
Orzolek A, Rafalska KT, Otowska WA, Kordan W, Korzekwa AJ, Kozłowski K (2021) Influence of Zinc and Manganese nanoparticles on selected parameters of turkey spermatozoa stored in a liquid state at 4°C. Animals 11:3289. https://doi.org/10.3390/ani11113289
Björndahl L, Kvist U (2009) Human sperm chromatin stabilization: a proposed model including zinc bridges. Mol Hum Reprod 16:23–29. https://doi.org/10.1093/molehr/gap099
Salmon TB, Evert BA, Song B, Doetsch PW (2004) Biological consequences of oxidative stress-induced DNA damage in Saccharomyces cerevisiae. Nucleic Acids Res 32:3712–3723. https://doi.org/10.1093/nar/gkh696
Lee SS, Song W, Cho M, Puppala HL, Nguyen P, Zhu H, Segatori L, Colvin VL (2013) Antioxidant properties of cerium oxide nanocrystals as a function of nanocrystal diameter and surface coating. ACS Nano 7:9693–9703. https://doi.org/10.1021/nn4026806
Flohe L (2007) Selenium in mammalian spermiogenesis. Biol Chem 388:987–995. https://doi.org/10.1515/BC.2007.112
Satorre MM, Breininger E, Beconi MT, Beorlegui NB (2007) α-Tocopherol modifies tyrosine phosphorylation and capacitation-like state of cryopreserved porcine sperm. Theriogenology 68:958–965. https://doi.org/10.1016/j.theriogenology.2007.06.021
Moce E, Purdy PH, Graham JK (2010) Treating ram sperm with cholesterol-loaded cyclodextrins improves cryosurvival. Anim Reprod Sci 18:236–247. https://doi.org/10.1016/j.anireprosci.2009.06.013
Longobardi V, Albero G, De Canditiis C, Salzano A, Natale A, Balestrieri A, Neglia G, Campanile G, Gasparrini B (2017) Cholesterol-loaded cyclodextrins prevent cryocapacitation damages in buffalo (Bubalus bubalis) cryopreserved sperm. Theriogenology 89:359–364. https://doi.org/10.1016/j.theriogenology.2016.09.048
Salmon VM, Leclerc P, Bailey JL (2016) Cholesterol-loaded cyclodextrin increases the cholesterol content of goat sperm to improve cold and osmotic resistance and maintain sperm function after cryopreservation. Biol Reprod 94:85–91. https://doi.org/10.1095/biolreprod.115.128553
Cormier N, Bailey JL (2003) A differential mechanism is involved during heparin and cryopreservation-induced capacitation of bovine spermatozoa. Biol Reprod 69:177–185. https://doi.org/10.1095/biolreprod.102.011056
Petrunkina AM, Volker G, Weitze KF, Beyerbach M, Topfer-Petersen E, Waberski D (2005) Detection of cooling-induced membrane changes in the response of boar sperm to capacitating conditions. Theriogenology 63:2278–2299. https://doi.org/10.1016/j.theriogenology.2004.10.008
Kim JG, Parthasarathy S (1998) Oxidation and the spermatozoa. Semin Reprod Endocrinol 16:235–339. https://doi.org/10.1055/s-2007-1016283
Agarwal A, Makker K, Sharma R (2008) Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 59:2–11. https://doi.org/10.1111/j.1600-0897.2007.00559.x
Fridovich I (1985) Superoxide dismutase, regularities and irregularities. Harvey Lect 79:51–75
Liu H, Sun Y, Zhao J, Dong W, Yang G (2020) Effect of zinc supplementation on semen quality, sperm antioxidant ability, and seminal and blood plasma mineral profiles in cashmere goats. Biol Trace Elem Res 196:438–445. https://doi.org/10.1007/s12011-019-01933-x
Zhang X, Wu H, Wu D, Wang Y, Chang J, Zhai Z, Meng A, Liu P, Zhang L, Fan F (2010) Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomed 5:771–781. https://doi.org/10.2147/IJN.S8428
Asri-Rezaei S, Nourian A, Shalizar-Jalali A, Najafi G, Nazarizadeh A, Koohestani M, Karimi A (2018) Selenium supplementation in the form of selenium nanoparticles and selenite sodium improves mature male mice reproductive performances. Iran J Basic Med Sci 21:577–585. https://doi.org/10.22038/IJBMS.2018.26023.6397
El-Maddawy Z, Abd El Naby WSH (2019) Protective effects of zinc oxide nanoparticles against doxorubicin induced testicular toxicity and DNA damage in male rats. Toxicol Res 8:654–662. https://doi.org/10.1039/c9tx00052f
Mammoto A, Masumoto N, Tahara M, Ikebuchi Y, Ohmichi M, Tasaka K, Miyake A (1996) Reactive oxygen species block sperm-egg fusion via oxidation of sperm sulfhydryl proteins in mice. Biol Reprod 55:1063–1068. https://doi.org/10.1095/biolreprod55.5.1063
Jones R, Mann T, Sherins R (1979) Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril 31:531–537. https://doi.org/10.1016/s0015-0282(16)43999-3
Mahboub HH, Shahin K, Zaglool AW, Roushdy EM, Ahmed SAA (2020) Efficacy of nano zinc oxide dietary supplements on growth performance, immunomodulation and disease resistance of African catfish Clarias gariepinus. Dis Aquat Organ 142:147–160. https://doi.org/10.1155/2022/8371440
Graham EF, Pace MM (1967) Some biochemical changes in spermatozoa due to freezing. Cryobiology 4:75–84. https://doi.org/10.1016/S0011-2240(67)80214-1
Upreti GC, Payne SR, Duganzich DM, Oliver JE, Smith JF (1996) Enzyme leakage during cryopreservation of ram spermatozoa. Anim Reprod Sci 41:27–36. https://doi.org/10.1016/0378-4320(95)01442-X
El-Harairy MA, Eid LN, Zeidan AEB, El-Salaam AA, El-Kishk MAM (2011) Quality and fertility of the frozen-thawed bull semen as affected by the different cryoprotectants and glutathione levels. J Am Sci 7:791–801
Reddy VS, Yadav B, Yadav CL, Anand M, Swain DK, Kumar D, Kritania D, Madan AK, Kumar J, Yadav S (2018) Effect of sericin supplementation on heat shock protein 70 (HSP70) expression, redox status and post thaw semen quality in goat. Cryobiology 84:33–39. https://doi.org/10.1016/j.cryobiol.2018.08.005
Patel KK, Kashfi K (2021) Lipoproteins and cancer: the role of HDL-C, LDL-C, and cholesterol-lowering drugs. Biochem Pharmacol 196:114654. https://doi.org/10.1016/j.bcp.2021.114654
Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, Agarwal A (2003) Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril 80:844–850. https://doi.org/10.1016/S0015-0282(03)00983-X
Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P (2002) Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod 17:1257–1265. https://doi.org/10.1093/humrep/17.5.1257
Gallon F, Marchetti C, Jouy N, Marchetti P (2006) The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil Steril 86:1526–1530. https://doi.org/10.1016/j.fertnstert.2006.03.055
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry and role in human disease. Am J Med 91:14–22. https://doi.org/10.1016/0002-9343(91)90279-7
Nichi M, Bols PEJ, Züge RM, Barnabe VH, Goovaerts IGF, Barnabe RC, Cortada CNM (2006) Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions. Theriogenology 66:822–828. https://doi.org/10.1016/j.theriogenology.2006.01.056
Lewis SE, Sterling ESL, Young IS, Thompson W (1997) Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril 67:142–147. https://doi.org/10.1016/s0015-0282(97)81871-7
Sharma RK, Agarwal A (1996) Role of reactive oxygen species in male infertility. Urology 48:835–850. https://doi.org/10.1016/S0090-4295(96)00313-5
Nagasaka R, Okamoto N, Ushio H (2004) Partial oxidative-stress perturbs membrane permeability and fluidity of fish nucleated red blood cells. Comp Biochem Physiol C Toxicol Pharmacol 139:259–266. https://doi.org/10.1016/j.cca.2004.12.001
Aitken RJ (1995) Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev 7:659–668. https://doi.org/10.1071/RD9950659
Gadea J, Molla M, Selles E, Marco MA, Garcia-Vazquez FA, Gardon JC (2011) Reduced glutathione content in human sperm is decreased after cryopreservation: effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology 62:40–46. https://doi.org/10.1016/j.cryobiol.2010.12.001
Salmani H, Towhidi A, Zhandi M, Bahreini M, Sharafi M (2014) In vitro assessment of soybean lecithin and egg yolk based diluents for cryopreservation of goat semen. Cryobiology 68:276–280. https://doi.org/10.1016/j.cryobiol.2014.02.008
Kumar P, Kumar D, Sikka P, Singh P (2015) Sericin supplementation improves semen freezability of buffalo bulls by minimizing oxidative stress during cryopreservation. Anim Reprod Sci 152:26–31. https://doi.org/10.1016/j.anireprosci.2014.11.015
Banday MN, Lone FA, Rasool F, Rashid M, Shikari A (2017) Use of antioxidants reduce lipid peroxidation and improve quality of crossbred ram sperm during its cryopreservation. Cryobiology 74:25–30. https://doi.org/10.1016/j.cryobiol.2016.12.008
Acknowledgements
The authors are grateful to the sophisticated analytical instrument facility (SAIF) of North Eastern Hill University for instrumental support in nanoparticle characterization. The authors also thankfully acknowledge the Director, Indian Council of Agricultural Research (ICAR), Research Complex for North Eastern Hill Region, for granting the necessary permissions to carry out the research work.
Funding
The work was supported by the Indian Council of Agricultural Research, New Delhi, India, through an in-house project.
Author information
Authors and Affiliations
Contributions
Anubha Baruah, Kishore Kumar Baruah, and Sourabh Deori conceptualized the experimental design, coordinated the study, discussed the results, and gave important contributions to the writing. Sayed Nabil Abedin performed the in vitro trials in the laboratory, compiled the results, did the statistical analyses, and compiled the draft version of the manuscript. Govindasamy Kadirvel and Rahul Katiyar supervised the work. Arundhati Bora, Devo Jyoti Dutta, Sudip Sinha, Shantanu Tamuly, and Arundhati Phookan formatted and edited the final manuscript version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Consent and Animal Use
A written approval of the Animal Ethics Committee of the institute was obtained prior to the onset of the experiment (770/GO/Re/S/03/CPCSEA/FVSc/AAU/IAEC/21–22/938).
Consent to Participate
NA
Consent for Publication
NA
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abedin, S.N., Baruah, A., Baruah, K.K. et al. In Vitro and In Vivo Studies on the Efficacy of Zinc-Oxide and Selenium Nanoparticle in Cryopreserved Goat (Capra hircus) Spermatozoa. Biol Trace Elem Res 201, 4726–4745 (2023). https://doi.org/10.1007/s12011-022-03551-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03551-6