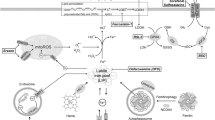
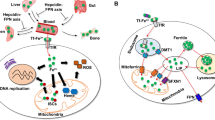
Abstract
Ferroptosis is a unique form of programmed cell death driven by iron-dependent phospholipid peroxidation that was proposed in recent years. It plays an important role in processes of various trace element-related diseases and is regulated by redox homeostasis and various cellular metabolic pathways (iron, amino acids, lipids, sugars), as well as disease-related signaling pathways. Some limited pioneering studies have demonstrated ferroptosis as a mechanism for the health effects of essential trace elements and potentially toxic trace elements, with crosstalk among them. The aim of this review is to bring together research articles and identify key direct and indirect evidence regarding essential trace elements (iron, selenium, zinc, copper, chromium, manganese) and potentially toxic trace elements (arsenic, aluminum, mercury) and their possible roles in ferroptosis. Our review may help determine future research priorities and opportunities.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Fraga CG, Oteiza PI, Keen CL. Trace elements and human health. Mol Aspects Med. 2005 Aug-Oct;26(4–5):233–4. PMID: 16122783. https://doi.org/10.1016/j.mam.2005.07.014
World Health Organization, International Atomic Energy Agency, Nations FaAOotU. Trace elements in human nutrition and health. Geneva: World Health Organization; 1996.
Cannas D, Loi E, Serra M, Firinu D, Valera P, Zavattari P (2020) Relevance of essential trace elements in nutrition and drinking water for human health and autoimmune disease risk. Nutrients. 12(7). https://doi.org/10.3390/nu12072074
Wagner A, Wang C, Fessler J, DeTomaso D, Avila-Pacheco J, Kaminski J et al (2021) Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell 184(16):4168–85 e21. https://doi.org/10.1016/j.cell.2021.05.045
Yang WS, Stockwell BR (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26(3):165–76. https://doi.org/10.1016/j.tcb.2015.10.014
Bayir H, Anthonymuthu TS, Tyurina YY, Patel SJ, Amoscato AA, Lamade AM et al (2020) Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem Biol 27(4):387–408. https://doi.org/10.1016/j.chembiol.2020.03.014
Dev S, Babitt JL (2017) Overview of iron metabolism in health and disease. Hemodial Int 21(1):S6–S20. https://doi.org/10.1111/hdi.12542
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117(3):285–97. https://doi.org/10.1016/s0092-8674(04)00343-5
Krzywoszynska K, Witkowska D, Swiatek-Kozlowska J, Szebesczyk A, Kozlowski H (2020) General aspects of metal ions as signaling agents in health and disease. Biomolecules 10(10):1417. https://doi.org/10.3390/biom10101417
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicol 283(2–3):65–87. https://doi.org/10.1016/j.tox.2011.03.001
Wolonciej M, Milewska E, Roszkowska-Jakimiec W (2016) Trace elements as an activator of antioxidant enzymes. Postepy Hig Med Dosw (Online) 70:1483–98. https://doi.org/10.5604/17322693.1229074
Conrad M, Angeli JP, Vandenabeele P, Stockwell BR (2016) Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 15(5):348–66. https://doi.org/10.1038/nrd.2015.6
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541. https://doi.org/10.1038/s41418-017-0012-4
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G (2019) The molecular machinery of regulated cell death. Cell Res 29(5):347–64. https://doi.org/10.1038/s41422-019-0164-5
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. N Engl J Med 361(16):1570–83. https://doi.org/10.1056/NEJMra0901217
Dolma S, Lessnick SL, Hahn WC, Stockwell BR (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3(3):285–96. https://doi.org/10.1016/s1535-6108(03)00050-3
Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ et al (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447(7146):864–8. https://doi.org/10.1038/nature05859
Yang WS, Stockwell BR (2008) Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15(3):234–45. https://doi.org/10.1016/j.chembiol.2008.02.010
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Chen X, Comish PB, Tang D, Kang R (2021) Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol 9:637162. https://doi.org/10.3389/fcell.2021.637162
Wang H, Liu C, Zhao Y, Gao G (2020) Mitochondria regulation in ferroptosis. Eur J Cell Biol. 99(1):151058. https://doi.org/10.1016/j.ejcb.2019.151058
Miyake S, Murai S, Kakuta S, Uchiyama Y, Nakano H (2020) Identification of the hallmarks of necroptosis and ferroptosis by transmission electron microscopy. Biochem Biophys Res Commun. 527(3):839–44. https://doi.org/10.1016/j.bbrc.2020.04.127
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–82. https://doi.org/10.1038/s41580-020-00324-8
Gao H, Bai Y, Jia Y, Zhao Y, Kang R, Tang D et al (2018) Ferroptosis is a lysosomal cell death process. Biochem Biophys Res Commun 503(3):1550–6. https://doi.org/10.1016/j.bbrc.2018.07.078
Dixon SJ, Stockwell BR (2019) The hallmarks of ferroptosis. Annu Rev Cancer Biol 3(1):35–54. https://doi.org/10.1146/annurev-cancerbio-030518-055844
Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X (2016) Ferroptosis is an autophagic cell death process. Cell Res 26(9):1021–1032. https://doi.org/10.1038/cr.2016.95
Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285. https://doi.org/10.1016/j.cell.2017.09.021
Kazan K, Kalaipandian S (2019) Ferroptosis: yet another way to die. Trends Plant Sci 24(6):479–81. https://doi.org/10.1016/j.tplants.2019.03.005
Green DR (2019) The coming decade of cell death research: five riddles. Cell 177(5):1094–107. https://doi.org/10.1016/j.cell.2019.04.024
Yuan H, Pratte J, Giardina C (2021) Ferroptosis and its potential as a therapeutic target. Biochem Pharmacol 186:114486. https://doi.org/10.1016/j.bcp.2021.114486
Tang D, Kroemer G (2020) Ferroptosis. Curr Biol 30(21):R1292–R7. https://doi.org/10.1016/j.cub.2020.09.068
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N et al (2020) Ferroptosis: past, present and future. Cell Death Dis 11(2):88. https://doi.org/10.1038/s41419-020-2298-2
Dixon SJ (2017) Ferroptosis: bug or feature? Immunol Rev 277(1):150–7. https://doi.org/10.1111/imr.12533
Sharma A, Flora SJS (2021) Positive and negative regulation of ferroptosis and its role in maintaining metabolic and redox homeostasis. Oxidative Med Cell Longev 2021:9074206. https://doi.org/10.1155/2021/9074206
Hao S, Liang B, Huang Q, Dong S, Wu Z, He W et al (2018) Metabolic networks in ferroptosis. Oncol Lett 15(4):5405–11. https://doi.org/10.3892/ol.2018.8066
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA et al (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther 6(1):49. https://doi.org/10.1038/s41392-020-00428-9
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X et al (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–79. https://doi.org/10.1038/cdd.2015.158
Tang M, Chen Z, Wu D, Chen L (2018) Ferritinophagy/ferroptosis: iron-related newcomers in human diseases. J Cell Physiol 233(12):9179–90. https://doi.org/10.1002/jcp.26954
Latunde-Dada GO (2017) Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj 1861(8):1893–900. https://doi.org/10.1016/j.bbagen.2017.05.019
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10(1):9–17. https://doi.org/10.1038/nchembio.1416
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X (2015) Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59(2):298–308. https://doi.org/10.1016/j.molcel.2015.06.011
Daher B, Vucetic M, Pouyssegur J (2020) Cysteine depletion, a key action to challenge cancer cells to ferroptotic cell death. Front Oncol 10:723. https://doi.org/10.3389/fonc.2020.00723
Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T (2017) Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol 403:143–70. https://doi.org/10.1007/82_2016_508
Alborzinia H, Ignashkova TI, Dejure FR, Gendarme M, Theobald J, Wolfl S et al (2018) Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun Biol 1:210. https://doi.org/10.1038/s42003-018-0212-6
Shojaie L, Iorga A, Dara L (2020) Cell death in liver diseases: a review. Int J Mol Sci 21(24). https://doi.org/10.3390/ijms21249682
Wu J, Wang Y, Jiang R, Xue R, Yin X, Wu M et al (2021) Ferroptosis in liver disease: new insights into disease mechanisms. Cell Death Discov 7(1):276. https://doi.org/10.1038/s41420-021-00660-4
Liu CY, Wang M, Yu HM, Han FX, Wu QS, Cai XJ et al (2020) Ferroptosis is involved in alcohol-induced cell death in vivo and in vitro. Biosci Biotechnol Biochem 84(8):1621–8. https://doi.org/10.1080/09168451.2020.1763155
Yamada N, Karasawa T, Kimura H, Watanabe S, Komada T, Kamata R et al (2020) Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis 11(2):144. https://doi.org/10.1038/s41419-020-2334-2
Yuan H, Li X, Zhang X, Kang R, Tang D (2016) CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun 478(2):838–44. https://doi.org/10.1016/j.bbrc.2016.08.034
Gao G, Xie Z, Li EW, Yuan Y, Fu Y, Wang P et al (2021) Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med 75(3):540–52. https://doi.org/10.1007/s11418-021-01491-4
Fang X, Wang H, Han D, Xie E, Yang X, Wei J et al (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A 116(7):2672–80. https://doi.org/10.1073/pnas.1821022116
Mahoney-Sanchez L, Bouchaoui H, Ayton S, Devos D, Duce JA, Devedjian JC (2021) Ferroptosis and its potential role in the physiopathology of Parkinson’s disease. Prog Neurobiol 196:101890. https://doi.org/10.1016/j.pneurobio.2020.101890
Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv H et al (2020) Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett 483:127–36. https://doi.org/10.1016/j.canlet.2020.02.015
Polo-Romero FJ (2006) Intramuscular deferoxamine in hereditary hemochromatosis. Am J Hematol 81(3):225–6. https://doi.org/10.1002/ajh.20450
Rustin P, Munnich A, Rotig A (1999) Quinone analogs prevent enzymes targeted in Friedreich ataxia from iron-induced injury in vitro. Biofactors 9(2–4):247–51. https://doi.org/10.1002/biof.5520090220
Calvaruso G, Vitrano A, Di Maggio R, Lai E, Colletta G, Quota A et al (2015) Deferiprone versus deferoxamine in thalassemia intermedia: results from a 5-year long-term Italian multicenter randomized clinical trial. Am J Hematol 90(7):634–8. https://doi.org/10.1002/ajh.24024
Xue Y, Zhang G, Zhou S, Wang S, Lv H, Zhou L, et al (2021) Iron chelator induces apoptosis in osteosarcoma cells by disrupting intracellular iron homeostasis and activating the MAPK pathway. Int J Mol Sci 22(13.) https://doi.org/10.3390/ijms22137168
Saha P, Yeoh BS, Xiao X, Golonka RM, Kumarasamy S, Vijay-Kumar M (2019) Enterobactin, an iron chelating bacterial siderophore, arrests cancer cell proliferation. Biochem Pharmacol 168:71–81. https://doi.org/10.1016/j.bcp.2019.06.017
Yu Y, Gutierrez E, Kovacevic Z, Saletta F, Obeidy P, SuryoRahmanto Y et al (2012) Iron chelators for the treatment of cancer. Curr Med Chem 19(17):2689–702. https://doi.org/10.2174/092986712800609706
Hassannia B, Vandenabeele P, VandenBerghe T (2019) Targeting ferroptosis to iron out cancer. Cancer Cell 35(6):830–49. https://doi.org/10.1016/j.ccell.2019.04.002
Liang C, Zhang X, Yang M, Dong X (2019) Recent progress in ferroptosis inducers for cancer therapy. Adv Mater 31(51):e1904197. https://doi.org/10.1002/adma.201904197
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C et al (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12(1):34. https://doi.org/10.1186/s13045-019-0720-y
Lieber CS, DeCarli LM (1968) Ethanol oxidation by hepatic microsomes: adaptive increase after ethanol feeding. Sci 162(3856):917–8. https://doi.org/10.1126/science.162.3856.917
Jiang Y, Zhang T, Kusumanchi P, Han S, Yang Z, Liangpunsakul S (2020) Alcohol metabolizing enzymes, microsomal ethanol oxidizing system, cytochrome P450 2E1, catalase, and aldehyde dehydrogenase in alcohol-associated liver disease. Biomedicines 8(3). https://doi.org/10.3390/biomedicines8030050
Cederbaum AI (1989) Oxygen radical generation by microsomes: role of iron and implications for alcohol metabolism and toxicity. Free Radical Biol Med 7(5):559–67. https://doi.org/10.1016/0891-5849(89)90033-6
Bacon BR, Healey JF, Brittenham GM, Park CH, Nunnari J, Tavill AS et al (1989) Hepatic microsomal function in rats with chronic dietary iron overload. Gastroenterol 90(6):1844–53. https://doi.org/10.1016/0016-5085(86)90251-9
Imam MU, Zhang S, Ma J, Wang H, Wang F (2017) Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients 9(7). https://doi.org/10.3390/nu9070671
Rana S, Prabhakar N (2021) Iron disorders and hepcidin. Clin Chim Acta 523:454–68. https://doi.org/10.1016/j.cca.2021.10.032
Kajarabille N, Latunde-Dada GO (2019) Programmed cell-death by ferroptosis: antioxidants as mitigators. Int J Mol Sci 20(19). https://doi.org/10.3390/ijms20194968
Shi H, Almutairi M, Moskovitz J, Xu YG (2021) Recent advances in iron homeostasis and regulation—a focus on epigenetic regulation and stroke. Free Radic Res 55(4):375–83. https://doi.org/10.1080/10715762.2020.1867314
Zeidan RS, Han SM, Leeuwenburgh C, Xiao R (2021) Iron homeostasis and organismal aging. Ageing Res Rev 72:101510. https://doi.org/10.1016/j.arr.2021.101510
Drakesmith H, Prentice AM (2012) Hepcidin and the iron-infection axis. Science 338(6108):768–72. https://doi.org/10.1126/science.1224577
Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC (1999) Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet 21(4):396–9. https://doi.org/10.1038/7727
Puntarulo S (2005) Iron, oxidative stress and human health. Mol Aspects Med 26(4–5):299–312. https://doi.org/10.1016/j.mam.2005.07.001
Koleini N, Shapiro JS, Geier J, Ardehali H (2021) Ironing out mechanisms of iron homeostasis and disorders of iron deficiency. J Clin Invest 131(11) https://doi.org/10.1172/JCI148671
Fu W, Yi J, Cheng M, Liu Y, Zhang G, Li L et al (2022) When bimetallic oxides and their complexes meet Fenton-like process. J Hazard Mater 424(Pt B):127419. https://doi.org/10.1016/j.jhazmat.2021.127419
Brown JB, Lee MA, Smith AT (2021) Ins and outs: recent advancements in membrane protein-mediated prokaryotic ferrous iron transport. Biochem 60(44):3277–91. https://doi.org/10.1021/acs.biochem.1c00586
Tang D, Chen X, Kang R, Kroemer G (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31(2):107–25. https://doi.org/10.1038/s41422-020-00441-1
Zhang Y, Fan BY, Pang YL, Shen WY, Wang X, Zhao CX et al (2020) Neuroprotective effect of deferoxamine on erastininduced ferroptosis in primary cortical neurons. Neural Regen Res 15(8):1539–45. https://doi.org/10.4103/1673-5374.274344
Peng Y, Chang X, Lang M (2021) Iron homeostasis disorder and Alzheimer's disease. Int J Mol Sci 22(22). https://doi.org/10.3390/ijms222212442
Leng Y, Luo X, Yu J, Jia H, Yu B (2021) Ferroptosis: a potential target in cardiovascular disease. Front Cell Dev Biol 9:813668. https://doi.org/10.3389/fcell.2021.813668
Chen S, Chen Y, Zhang Y, Kuang X, Liu Y, Guo M et al (2020) Iron metabolism and ferroptosis in epilepsy. Front Neurosci 14:601193. https://doi.org/10.3389/fnins.2020.601193
Stockwell BR, Jiang X, Gu W (2020) Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol 30(6):478–90. https://doi.org/10.1016/j.tcb.2020.02.009
Chen J, Li X, Ge C, Min J, Wang F (2022) The multifaceted role of ferroptosis in liver disease. Cell Death Differ 29(3):467–80. https://doi.org/10.1038/s41418-022-00941-0
Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P et al (2020) Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 136(6):726–39. https://doi.org/10.1182/blood.2019002907
Wu A, Feng B, Yu J, Yan L, Che L, Zhuo Y et al (2021) Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol 46:102131. https://doi.org/10.1016/j.redox.2021.102131
Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P et al (2020) Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res 127(4):486–501. https://doi.org/10.1161/CIRCRESAHA.120.316509
Conrad M, Proneth B (2019) Broken hearts: iron overload, ferroptosis and cardiomyopathy. Cell Res 29(4):263–4. https://doi.org/10.1038/s41422-019-0150-y
Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN (2019) Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev 99(4):1765–817. https://doi.org/10.1152/physrev.00022.2018
Zhang Y, Xin L, Xiang M, Shang C, Wang Y, Wang Y et al (2022) The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed Pharmacother 145:112423. https://doi.org/10.1016/j.biopha.2021.112423
Wu X, Li Y, Zhang S, Zhou X (2021) Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 11(7):3052–9. https://doi.org/10.7150/thno.54113
Bai Q, Liu J, Wang G (2020) Ferroptosis, a regulated neuronal cell death type after intracerebral hemorrhage. Front Cell Neurosci 14:591874. https://doi.org/10.3389/fncel.2020.591874
Gleason A, Bush AI (2021) Iron and ferroptosis as therapeutic targets in Alzheimer’s disease. Neurotherapeutics 18(1):252–64. https://doi.org/10.1007/s13311-020-00954-y
Rayman MP (2012) Selenium and human health. The Lancet 379(9822):1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Hatfield DL, Berry MJ, Gladyshev VN (2011) Selenium: its molecular biology and role in human health: Springer Science & Business Media ISBN: 1461410258.
Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE et al (2011) Selenium in human health and disease. Antioxid Redox Signal 14(7):1337–83. https://doi.org/10.1089/ars.2010.3275
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9(7):775–806. https://doi.org/10.1089/ars.2007.1528
Tinggi U (2008) Selenium: its role as antioxidant in human health. Environ Health Prev Med. 13(2):102–8. https://doi.org/10.1007/s12199-007-0019-4
Zoidis E, Seremelis I, Kontopoulos N, Danezis GP (2018) Selenium-dependent antioxidant enzymes: actions and properties of selenoproteins. Antioxidants (Basel) 7(5) https://doi.org/10.3390/antiox7050066
Tsuji PA, Santesmasses D, Lee BJ, Gladyshev VN, Hatfield DL (2021) Historical roles of selenium and selenoproteins in health and development: the good, the bad and the ugly. Int J Mol Sci. 23(1) https://doi.org/10.3390/ijms23010005
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–41. https://doi.org/10.1016/S0140-6736(00)02490-9
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54. https://doi.org/10.1039/c3mt00185g
Santesmasses D, Gladyshev VN (2021) Pathogenic variants in selenoproteins and selenocysteine biosynthesis machinery. Int J Mol Sci 22(21) https://doi.org/10.3390/ijms222111593
Bulteau AL, Chavatte L (2015) Update on selenoprotein biosynthesis. Antioxid Redox Signal 23(10):775–94. https://doi.org/10.1089/ars.2015.6391
Labunskyy VM, Hatfield DL, Gladyshev VN (2014) Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94(3):739–77. https://doi.org/10.1152/physrev.00039.2013
Brown KM, Selenium Arthur JR. (2001) selenoproteins and human health: a review. Public Health Nutr 4(2B):593–9. https://doi.org/10.1079/phn2001143
Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochimica et Biophysica Acta (BBA)-General Subjects 1830(5):3289–303. https://doi.org/10.1016/j.bbagen.2012.11.020
Gromer S, Eubel J, Lee B, Jacob J (2005) Human selenoproteins at a glance. Cell Mol Life Sci CMLS 62(21):2414–2437. https://doi.org/10.1007/s00018-005-5143-y
Lennarz WJ, Lane MD (2013) Encyclopedia of biological chemistry: Academic Press
Bridges RJ, Natale NR, Patel SA (2012) System xc-cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 165(1):20–34. https://doi.org/10.1111/j.1476-5381.2011.01480.x
Scirè A, Cianfruglia L, Minnelli C, Bartolini D, Torquato P, Principato G et al (2019) Glutathione compartmentalization and its role in glutathionylation and other regulatory processes of cellular pathways. BioFactors 45(2):152–168. https://doi.org/10.1002/biof.1476
Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E et al (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262–79 e25. https://doi.org/10.1016/j.cell.2019.03.032
Li C, Deng X, Zhang W, Xie X, Conrad M, Liu Y et al (2019) Novel allosteric activators for ferroptosis regulator glutathione peroxidase 4. J Med Chem 62(1):266–75. https://doi.org/10.1021/acs.jmedchem.8b00315
Li P, Jiang M, Li K, Li H, Zhou Y, Xiao X et al (2021) Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol 22(9):1107–17. https://doi.org/10.1038/s41590-021-00993-3
Thayyullathil F, Cheratta AR, Alakkal A, Subburayan K, Pallichankandy S, Hannun YA et al (2021) Acid sphingomyelinase-dependent autophagic degradation of GPX4 is critical for the execution of ferroptosis. Cell Death Dis 12(1):26. https://doi.org/10.1038/s41419-020-03297-w
Park TJ, Park JH, Lee GS, Lee JY, Shin JH, Kim MW et al (2019) Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis 10(11):835. https://doi.org/10.1038/s41419-019-2061-8
Seibt TM, Proneth B, Conrad M (2019) Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med 133:144–52. https://doi.org/10.1016/j.freeradbiomed.2018.09.014
Cao JY, Dixon SJ (2016) Mechanisms of ferroptosis. Cell Mol Life Sci 73(11–12):2195–209. https://doi.org/10.1007/s00018-016-2194-1
Poltorack CD, Dixon SJ (2022) Understanding the role of cysteine in ferroptosis: progress & paradoxes. FEBS J 289(2):374–85. https://doi.org/10.1111/febs.15842
Kim SJ, Choi MC, Park JM, Chung AS (2021) Antitumor effects of selenium. Int J Mol Sci 22(21):11844. https://doi.org/10.3390/ijms222111844
Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K et al (2018) Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172(3):409–22 e21. https://doi.org/10.1016/j.cell.2017.11.048
Cardoso BR, Hare DJ, Bush AI, Roberts BR (2017) Glutathione peroxidase 4: a new player in neurodegeneration? Mol Psychiatry 22(3):328–35. https://doi.org/10.1038/mp.2016.196
Zhang X, Huang Z, Xie Z, Chen Y, Zheng Z, Wei X et al (2020) Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med 160:552–65. https://doi.org/10.1016/j.freeradbiomed.2020.08.029
Cunha TA, Vermeulen-Serpa KM, Grilo EC, Leite-Lais L, Brandão-Neto J, Vale SH (2022) Association between zinc and body composition: an integrative review. J Trace Elem Med Biol 71:126940. https://doi.org/10.1016/j.jtemb.2022.12694
Scavo S, Oliveri V (2021) Zinc ionophores: chemistry and biological applications. J Inorg Biochem 228:111691. https://doi.org/10.1016/j.jinorgbio.2021.111691
Ge MH, Tian H, Mao L, Li DY, Lin JQ, Hu HS, et al (2021) Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci Ther https://doi.org/10.1111/cns.13657
Ho E, Wong CP, King JC (2022) Impact of zinc on DNA integrity and age-related inflammation. Free Radic Biol Med 178:391–7. https://doi.org/10.1016/j.freeradbiomed.2021.12.256
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7(4):1342–65. https://doi.org/10.3390/ijerph7041342
Planeta Kepp K (2021) Bioinorganic chemistry of zinc in relation to the Immune system. Chem Bio Chem 23(9):e202100554. https://doi.org/10.1002/cbic.202100554
Li X, Han M, Zhang H, Liu F, Pan Y, Zhu J et al (2022) Structures and biological functions of zinc finger proteins and their roles in hepatocellular carcinoma. Biomarker Res 10(1):1–13. https://doi.org/10.1186/s40364-021-00345-1
Roohani N, Hurrell R, Kelishadi R, Schulin R (2013) Zinc and its importance for human health: an integrative review. J Res Med Sci 18(2):144
Wandzilak A, Czyzycki M, Wrobel P, Szczerbowska-Boruchowska M, Radwanska E, Adamek D et al (2013) The oxidation states and chemical environments of iron and zinc as potential indicators of brain tumour malignancy grade–preliminary results. Metallomics 5(11):1547–1553. https://doi.org/10.1039/c3mt00158j
Baarz BR, Rink L (2021) Rebalancing the unbalanced aged immune system-a special focus on zinc. Ageing Res Rev 74:101541. https://doi.org/10.1016/j.arr.2021.101541
Prasad AS (2014) Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr 1:14. https://doi.org/10.3389/fnut.2014.00014
Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zinc and human health: an update. Arch Toxicol 86(4):521–534. https://doi.org/10.1007/s00204-011-0775-1
Marreiro DDN, Cruz KJC, Morais JBS, Beserra JB, Severo JS, De Oliveira ARS (2017) Zinc and oxidative stress: current mechanisms. Antioxidants 6(2):24. https://doi.org/10.3390/antiox6020024
Ge Mh, Tian H, Mao L, Dy Li, Lin Jq, Hu Hs et al (2021) Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci Ther 27(9):1023–40. https://doi.org/10.1111/cns.13657
Tapiero H, Townsend DM, Tew KD (2003) Trace elements in human physiology and pathology. Copper. Biomed Pharmacother 57(9):386–98. https://doi.org/10.1016/s0753-3322(03)00012-x
da Silva DA, De Luca A, Squitti R, Rongioletti M, Rossi L, Machado CML et al (2022) Copper in tumors and the use of copper-based compounds in cancer treatment. J Inorg Biochem 226:111634. https://doi.org/10.1016/j.jinorgbio.2021.111634
Angelova M, Asenova S, Nedkova V, Koleva-Kolarova R (2011). Copper in the human organism. Trakia Trakia J Sci 9(1):88–98
Vetlényi E, Rácz G (2020) The physiological function of copper, the etiological role of copper excess and deficiency. Orv Hetil 161(35):1488–1496. https://doi.org/10.1556/650.2020.31854
Healy J, Tipton K (2007) Ceruloplasmin and what it might do. J Neural Transm (Vienna). 114(6):777–81. https://doi.org/10.1007/s00702-007-0687-7
Hellman NE, Gitlin JD (2002) Ceruloplasmin metabolism and function. Annu Rev Nutr. 22:439–58. https://doi.org/10.1146/annurev.nutr.22.012502.114457
Frieden E, Hsieh HS (1976) Ceruloplasmin: the copper transport protein with essential oxidase activity. Adv Enzymol Relat Areas Mol Biol 44:187–236. https://doi.org/10.1002/9780470122891.ch6
Lutsenko S (2021) Dynamic and cell-specific transport networks for intracellular copper ions. J Cell Sci 134(21):240523. https://doi.org/10.1242/jcs.240523
Rydén L (2018) Ceruloplasmin. CRC Press, Copper proteins and copper enzymes, pp 37–100
Pak K, Ordway S, Sadowski B, Canevari M, Torres D (2021) Wilson’s disease and iron overload: pathophysiology and therapeutic implications. Clin Liver Dis (Hoboken) 17(2):61–6. https://doi.org/10.1002/cld.986
Shang Y, Luo M, Yao F, Wang S, Yuan Z, Yang Y (2020) Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. Cell Signal 72:109633. https://doi.org/10.1016/j.cellsig.2020.109633
Balachandran RC, Mukhopadhyay S, McBride D, Veevers J, Harrison FE, Aschner M et al (2020) Brain manganese and the balance between essential roles and neurotoxicity. J Biol Chem 295(19):6312–29. https://doi.org/10.1074/jbc.REV119.009453
Ye Q, Park JE, Gugnani K, Betharia S, Pino-Figueroa A, Kim J (2017) Influence of iron metabolism on manganese transport and toxicity. Metallomics 9(8):1028–46. https://doi.org/10.1039/c7mt00079k
Bjorklund G, Aaseth J, Skalny AV, Suliburska J, Skalnaya MG, Nikonorov AA et al (2017) Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J Trace Elem Med Biol 41:41–53. https://doi.org/10.1016/j.jtemb.2017.02.005
Kwik-Uribe C, Smith DR (2006) Temporal responses in the disruption of iron regulation by manganese. J Neurosci Res 83(8):1601–10. https://doi.org/10.1002/jnr.20836
Pang L, Wang J, Huang W, Guo S (2015) A study of divalent metal transporter 1 and ferroportin 1 in brain of rats with manganese-induced parkinsonism. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 33(4):250–254
Achmad RT, Auerkari EI (2017) Effects of chromium on human body. Annual Research & Review in Biology 1–8
Zafra-Stone S, Bagchi M, Preuss H, Bagchi D (2007) Benefits of chromium (III) complexes in animal and human health. Nutr Biochem Chromium (III) 183–206
Chatterjee SJIJoAR (2015) Chromium Toxicity and its Health Hazards 3(7):167–72
Pellerin C, Booker SM (2000) Reflections on hexavalent chromium: health hazards of an industrial heavyweight. Environ Health Perspect 108(9):A402-7. https://doi.org/10.1289/ehp.108-a402
Prasad S, Yadav KK, Kumar S, Gupta N, Cabral-Pinto MMS, Rezania S et al (2021) Chromium contamination and effect on environmental health and its remediation: a sustainable approaches. J Environ Manage 285:112174. https://doi.org/10.1016/j.jenvman.2021.112174
Godwill EA, Ferdinand PU, Nweke FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. In: Karcioglu O, Arslan B (eds) Poisoning in the Modern World - New Tricks for an Old Dog?. IntechOpen. https://doi.org/10.5772/intechopen.82511
Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK (2021) Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 262:128350. https://doi.org/10.1016/j.chemosphere.2020.128350
Zeng Q, Zou Z, Wang Q, Sun B, Liu Y, Liang B et al (2019) Association and risk of five miRNAs with arsenic-induced multiorgan damage. Sci Total Environ 680:1–9. https://doi.org/10.1016/j.scitotenv.2019.05.042
Palma-Lara I, Martínez-Castillo M, Quintana-Pérez J, Arellano-Mendoza M, Tamay-Cach F, Valenzuela-Limón O et al (2020) Arsenic exposure: a public health problem leading to several cancers. Regul Toxicol Pharmacol 110:104539
Zeng Q, Zhang A (2020) Assessing potential mechanisms of arsenic-induced skin lesions and cancers: Human and in vitro evidence. Environ Pollut Barking Essex: 1987 260:113919. https://doi.org/10.1016/j.envpol.2020.113919
Ahmad S, Kitchin KT, Cullen WR (2000) Arsenic species that cause release of iron from ferritin and generation of activated oxygen. Arch Biochem Biophys 382(2):195–202. https://doi.org/10.1006/abbi.2000.2023
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D et al (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31(2):95–107. https://doi.org/10.1002/jat.1649
Carter DE (1995) Oxidation-reduction reactions of metal ions. Environ Health Perspect 103(1):17–9. https://doi.org/10.1289/ehp.95103s117
Wang Y, Liu Y, Liu S, Wu B (2019) Influence of iron on cytotoxicity and gene expression profiles induced by arsenic in HepG2 cells. Int J Environ Res Public Health 16(22):4484. https://doi.org/10.3390/ijerph16224484
Yan AN, Chun-Chun LI, Deng HYJFMS (2015) Current conditions of researches in arsenic-induced oxidative stress. Foreign Medical Sciences (Section of Medgeography) 3:165–173. https://doi.org/10.3969/j.issn.1001-8883.2015.03.001
Wei S, Qiu T, Wang N, Yao X, Jiang L, Jia X et al (2020) Ferroptosis mediated by the interaction between Mfn2 and IREα promotes arsenic-induced nonalcoholic steatohepatitis. Environ Res 188:109824. https://doi.org/10.1016/j.envres.2020.109824
Tang Q, Bai L, Zou Z, Meng P, Xia Y, Cheng S et al (2018) Ferroptosis is newly characterized form of neuronal cell death in response to arsenite exposure. Neurotoxicol 67:27–36. https://doi.org/10.1016/j.neuro.2018.04.012
Xiao J, Zhang S, Tu B, Jiang X, Cheng S, Tang Q et al (2021) Arsenite induces ferroptosis in the neuronal cells via activation of ferritinophagy. Food Chem Toxicol 151:112114. https://doi.org/10.1016/j.fct.2021.112114
Meng P, Zhang S, Jiang X, Cheng S, Zhang J, Cao X et al (2020) Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol Environ Saf 194:110360. https://doi.org/10.1016/j.ecoenv.2020.110360
Wei S, Qiu T, Yao X, Wang N, Jiang L, Jia X et al (2020) Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J Hazard Mater 384:121390. https://doi.org/10.1016/j.jhazmat.2019.121390
Lanser L, Fuchs D, Kurz K, Weiss G (2021) Physiology and inflammation driven pathophysiology of iron homeostasis—mechanistic insights into anemia of inflammation and its treatment. Nutrients 13(11):3732. https://doi.org/10.3390/nu13113732
Hyman MH (2004) The impact of mercury on human health and the environment. Altern Ther Health Med 10(6):70–5
Beckers F, Rinklebe J (2017) Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit Rev Env Sci Tec 47(9):93–794. https://doi.org/10.1080/10643389.2017.1326277
Kim KH, Kabir E, Jahan SA (2016) A review on the distribution of Hg in the environment and its human health impacts. J Hazard Mater 5(306):376–85. https://doi.org/10.1016/j.jhazmat.2015.11.031
Martins AC, Ke T, Bowman AB, Aschner M (2021) New insights on mechanisms underlying methylmercury-induced and manganese-induced neurotoxicity. Curr Opin Toxicol 25:30–5. https://doi.org/10.1016/j.cotox.2021.03.002
Ahmad S, Mahmood R (2019) Mercury chloride toxicity in human erythrocytes: enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ Sci Pollut Res Int 26(6):5645–57. https://doi.org/10.1007/s11356-018-04062-5
Chang J, Yang B, Zhou Y, Yin C, Liu T, Qian H et al (2019) Acute methylmercury exposure and the hypoxia-inducible factor-1alpha signaling pathway under normoxic conditions in the rat brain and astrocytes in vitro. Environ Health Perspect 127(12):127006. https://doi.org/10.1289/EHP5139
Liu T, Gao Q, Yang B, Yin C, Chang J, Qian H et al (2020) Differential susceptibility of PC12 and BRL cells and the regulatory role of HIF-1alpha signaling pathway in response to acute methylmercury exposure under normoxia. Toxicol Lett 331:82–91. https://doi.org/10.1016/j.toxlet.2020.05.023
Liu Y, Zhu W, Ni D, Zhou Z, Gu JH, Zhang W et al (2020) Alpha lipoic acid antagonizes cytotoxicity of cobalt nanoparticles by inhibiting ferroptosis-like cell death. J Nanobiotechnol 18(1):141. https://doi.org/10.1186/s12951-020-00700-8
Zhang Y, Zhang P, Li Y (2020) Gut microbiota-mediated ferroptosis contributes to mercury exposure-induced brain injury in common carp. Metallomics 14(1) https://doi.org/10.1093/mtomcs/mfab072
Antunes Dos Santos A, Ferrer B, Marques Goncalves F, Tsatsakis AM, Renieri EA, Skalny AV, et al (2018) Oxidative stress in methylmercury-induced cell toxicity. Toxics 6(3) https://doi.org/10.3390/toxics6030047
Kumar A, Khushboo Pandey R, Sharma B (2020) Modulation of superoxide dismutase activity by mercury, lead, and arsenic. Biol Trace Elem Res 196(2):654–61. https://doi.org/10.1007/s12011-019-01957-3
Ghizoni H, de Souza V, Straliotto MR, de Bem AF, Farina M, Hort MA (2017) Superoxide anion generation and oxidative stress in methylmercury-induced endothelial toxicity in vitro. Toxicol In Vitro 38:19–26. https://doi.org/10.1016/j.tiv.2016.10.010
Teschke R (2022) Aluminum, arsenic, beryllium, cadmium, chromium, cobalt, copper, iron, lead, mercury, molybdenum, nickel, platinum, thallium, titanium, vanadium, and zinc: molecular aspects in experimental liver injury. Int J Mol Sci 23(20 https://doi.org/10.3390/ijms232012213
Funding
This work was supported by the National Natural Science Foundations of China (82003402, 81860569), Guizhou Province Science and Technology Plan Project of China (No. ZK[2021] key 009) and the Excellent Young Talents Plan of Guizhou Medical University (No. [2021]102).
Author information
Authors and Affiliations
Contributions
Yuyan Xu: data collection, data analysis, data curation, writing—original draft, writing—review and editing, visualization, and funding acquisition. Ruobi Chen: data collection, data analysis, data curation, and writing—original draft. Qibing Zeng: design, resources, writing—review and editing, supervision, project administration, and funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Chen, R. & Zeng, Q. Ferroptosis As a Mechanism for Health Effects of Essential Trace Elements and Potentially Toxic Trace Elements. Biol Trace Elem Res 201, 4262–4274 (2023). https://doi.org/10.1007/s12011-022-03523-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03523-w