Abstract
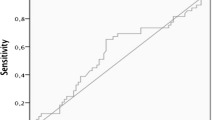
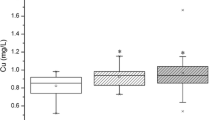
Magnesium is considered to play a role in preventing cancer. However, the association between serum magnesium and papillary thyroid cancer (PTC) remains unknown. We retrospectively reviewed records of all patients who underwent thyroidectomy with thyroid nodules confirmed pathologically as benign nodule or PTC at our institution from January 2016 to December 2020. Data including demographic characteristics, laboratory tests, and pathological features were analyzed in 5709 adult patients eventually. The subjects with benign nodules had a higher mean serum magnesium level than those with PTC (P < 0.001), and the proportions of PTCs decreased across quartiles of serum magnesium within the normal range. After adjustment for confounders, patients with the lowest quartile of serum magnesium had a higher prevalence of PTC than those with the highest quartile (OR = 1.421, 95%CI: 1.125–1.795, P for trend = 0.005), and the risk of PTC was 0.863 (95%CI: 0.795–0.936) for a per-SD change in serum magnesium. The contribution of serum magnesium remained in subgroup analysis (P for interaction for all analyses > 0.05). Based on the ROC curve, the cut-off value of serum magnesium used to differentiate benign nodules from PTCs was 935 μmol/L. Combining serum magnesium with other clinical indicators can improve the efficacy of predicting PTC. Our results showed that lower serum magnesium within the normal range was associated with a greater risk of PTC among patients with thyroid nodules considering thyroidectomy. Serum magnesium may be an independent protective factor against PTC and provide additional information on the odds of malignancy in uncertain thyroid nodules in combination with other clinical factors.


Similar content being viewed by others
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Kim J, Gosnell JE, Roman SA (2020) Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol 16(1):17–29. https://doi.org/10.1038/s41574-019-0263-x
Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM (2017) Trends in thyroid cancer incidence and mortality in the united states, 1974–2013. JAMA 317(13):1338–1348. https://doi.org/10.1001/jama.2017.2719
Morris LG, Myssiorek D (2010) Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 200(4):454–461. https://doi.org/10.1016/j.amjsurg.2009.11.008
Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS (2018) The diagnosis and management of thyroid nodules: a review. JAMA 319(9):914–924. https://doi.org/10.1001/jama.2018.0898
de Baaij JH, Hoenderop JG, Bindels RJ (2015) Magnesium in man: implications for health and disease. Physiol Rev 95(1):1–46. https://doi.org/10.1152/physrev.00012.2014
Dai Q, Motley SS, Smith JA Jr et al (2011) Blood magnesium, and the interaction with calcium, on the risk of high-grade prostate cancer. PLoS One 6(4):e18237. https://doi.org/10.1371/journal.pone.0018237
Polter EJ, Onyeaghala G, Lutsey PL et al (2019) Prospective association of serum and dietary magnesium with colorectal cancer incidence. Cancer Epidemiol Biomarkers Prev 28(8):1292–1299. https://doi.org/10.1158/1055-9965.Epi-18-1300
Yu G, Hsu WL, Coghill AE et al (2019) Whole-exome sequencing of nasopharyngeal carcinoma families reveals novel variants potentially involved in nasopharyngeal carcinoma. Sci Rep 9(1):9916. https://doi.org/10.1038/s41598-019-46137-4
Shahy EM, Taha MM, Ibrahim KS (2020) Assessment of ykl-40, lipid profile, antioxidant status, and some trace elements in benign and malignant breast proliferation. Mol Biol Rep 47(9):6973–6982. https://doi.org/10.1007/s11033-020-05756-1
Bezerra DLC, Mendes PMV, Melo SRS et al (2021) Hypomagnesemia and its relationship with oxidative stress markers in women with breast cancer. Biol Trace Elem Res 199(12):4466–4474. https://doi.org/10.1007/s12011-021-02579-4
Meng Y, Sun J, Yu J, Wang C, Su J (2019) Dietary intakes of calcium, iron, magnesium, and potassium elements and the risk of colorectal cancer: a meta-analysis. Biol Trace Elem Res 189(2):325–335. https://doi.org/10.1007/s12011-018-1474-z
Song X, Zhong X, Tang K, Wu G, Jiang Y (2018) Serum magnesium levels and lung cancer risk: a meta-analysis. World J Surg Oncol 16(1):137. https://doi.org/10.1186/s12957-018-1447-x
Leung PL, Li XL (1996) Multielement analysis in serum of thyroid cancer patients before and after a surgical operation. Biol Trace Elem Res 51(3):259–266. https://doi.org/10.1007/bf02784080
Al-Sayer H, Mathew TC, Asfar S et al (2004) Serum changes in trace elements during thyroid cancers. Mol Cell Biochem 260(1–2):1–5. https://doi.org/10.1023/b:mcbi.0000026027.20680.c7
Xing M (2012) Oxidative stress: a new risk factor for thyroid cancer. Endocr Relat Cancer 19(1):C7-11. https://doi.org/10.1530/erc-11-0360
Lassoued S, Mseddi M, Mnif F et al (2010) A comparative study of the oxidative profile in graves’ disease, Hashimoto’s thyroiditis, and papillary thyroid cancer. Biol Trace Elem Res 138(1–3):107–115. https://doi.org/10.1007/s12011-010-8625-1
Wang D, Feng JF, Zeng P, Yang YH, Luo J, Yang YW (2011) Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr Relat Cancer 18(6):773–782. https://doi.org/10.1530/erc-11-0230
Ameziane El Hassani R, Buffet C, Leboulleux S, Dupuy C (2019) Oxidative stress in thyroid carcinomas: Biological and clinical significance. Endocr Relat Cancer 26(3):R131-r143. https://doi.org/10.1530/erc-18-0476
Jia X, Pang P, Wang L et al (2020) Clinical analysis of preoperative anti-thyroglobulin antibody in papillary thyroid cancer between 2011 and 2015 in Beijing, China: a retrospective study. Front Endocrinol (Lausanne) 11:452. https://doi.org/10.3389/fendo.2020.00452
Kim ES, Lim DJ, Baek KH et al (2010) Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid 20(8):885–891. https://doi.org/10.1089/thy.2009.0384
Rago T, Fiore E, Scutari M et al (2010) Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol 162(4):763–770. https://doi.org/10.1530/eje-09-0895
Schmid D, Ricci C, Behrens G, Leitzmann MF (2015) Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev 16(12):1042–1054. https://doi.org/10.1111/obr.12321
Haymart MR, Repplinger DJ, Leverson GE et al (2008) Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 93(3):809–814. https://doi.org/10.1210/jc.2007-2215
Wang K, Wei H, Zhang W et al (2018) Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: a cross-sectional study. Sci Rep 8(1):9904. https://doi.org/10.1038/s41598-018-28362-5
Ehlers M, Schott M (2014) Hashimoto’s thyroiditis and papillary thyroid cancer: Are they immunologically linked? Trends Endocrinol Metab 25(12):656–664. https://doi.org/10.1016/j.tem.2014.09.001
Uhliarova B, Hajtman A (2018) Hashimoto’s thyroiditis - an independent risk factor for papillary carcinoma. Braz J Otorhinolaryngol 84(6):729–735. https://doi.org/10.1016/j.bjorl.2017.08.012
Pan J, Ye F, Yu C et al (2021) Papillary thyroid carcinoma landscape and its immunological link with hashimoto thyroiditis at single-cell resolution. Front Cell Dev Biol 9:758339. https://doi.org/10.3389/fcell.2021.758339
Hartwig A (2001) Role of magnesium in genomic stability. Mutat Res 475(1–2):113–121. https://doi.org/10.1016/s0027-5107(01)00074-4
Yang L, Arora K, Beard WA, Wilson SH, Schlick T (2004) Critical role of magnesium ions in DNA polymerase beta’s closing and active site assembly. J Am Chem Soc 126(27):8441–8453. https://doi.org/10.1021/ja049412o
Wolf FI, Maier JA, Nasulewicz A et al (2007) Magnesium and neoplasia: from carcinogenesis to tumor growth and progression or treatment. Arch Biochem Biophys 458(1):24–32. https://doi.org/10.1016/j.abb.2006.02.016
Morais JB, Severo JS, Santos LR et al (2017) Role of magnesium in oxidative stress in individuals with obesity. Biol Trace Elem Res 176(1):20–26. https://doi.org/10.1007/s12011-016-0793-1
Klatka M, Grywalska E, Partyka M, Charytanowicz M, Rolinski J (2013) Impact of methimazole treatment on magnesium concentration and lymphocytes activation in adolescents with graves’ disease. Biol Trace Elem Res 153(1–3):155–170. https://doi.org/10.1007/s12011-013-9690-z
Laughlin MR, Thompson D (1996) The regulatory role for magnesium in glycolytic flux of the human erythrocyte. J Biol Chem 271(46):28977–28983. https://doi.org/10.1074/jbc.271.46.28977
Kostov K (2019) Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci 20(6). https://doi.org/10.3390/ijms20061351
Pazaitou-Panayiotou K, Polyzos SA, Mantzoros CS (2013) Obesity and thyroid cancer: epidemiologic associations and underlying mechanisms. Obes Rev 14(12):1006–1022. https://doi.org/10.1111/obr.12070
Yin DT, He H, Yu K et al (2018) The association between thyroid cancer and insulin resistance, metabolic syndrome and its components: a systematic review and meta-analysis. Int J Surg 57:66–75. https://doi.org/10.1016/j.ijsu.2018.07.013
Acknowledgements
The authors thank the staff of the First Medical Center of Chinese PLA General Hospital, Beijing, China, for their participation in this study.
Author information
Authors and Affiliations
Contributions
Conception and design: Zhaohui Lyu. Material preparation: Xiaomeng Jia. Data collection: Huaijin Xu, Xiaodong Hu, Jiefei Li, Zhimei Nie, and Shaoyang Kang. Analysis and interpretation of data: Huaijin Xu. Validation: Xiaodong Hu, Hongzhou Liu, and Yuhan Wang.
Writing the original draft: Huaijin Xu. Revising the manuscript: Zhaohui Lyu.
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted in line with the Helsinki Declaration and approved by the Ethics Committee of Chinese PLA General Hospital (No.S2022-0115–01) with waived informed consent considering the collection of de-identified retrospective data.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, H., Hu, X., Li, J. et al. The Inverse Association of Serum Magnesium with Papillary Thyroid Cancer in Thyroid Nodules: a Cross-Sectional Survey Based on Thyroidectomy Population. Biol Trace Elem Res 201, 3279–3289 (2023). https://doi.org/10.1007/s12011-022-03448-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03448-4