Abstract
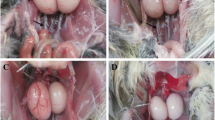
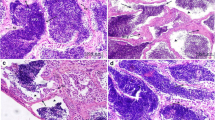
Lead (Pb) becomes a global public health concern for its high toxicology. Birds are sensitive to environmental pollution and Pb contamination exerts multiple negative influences on bird life. Pb also impacts on avian reproductive system. Thus, in this study, we attempted to determine toxicological effects and possible mechanistic pathways of Pb on avian testicular development by using the model species—Japanese quail (Coturnix japonica). Male quail chicks of 1-week-old were exposed to 0, 50, 500, and 1000 ppm Pb concentrations in drinking water for 5 weeks when reaching sexual maturation. The results showed that high Pb doses (500 and 1000 ppm) induced testis atrophy and cloacal gland shrinkage. Microstructural damages of both hypothalamus and testis indicated the disruption of the hypothalamus-pituitary–gonadal (HPG) axis by Pb exposure. The decrease of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and testosterone (T) may also imply HPG axis disruption. Moreover, excess testicular oxidative damages featured by increasing reactive oxygen species (ROS) and malondialdehyde (MDA) and decreasing catalase (CAT), glutathione (GSH), superoxide dismutase (SOD), glutathione-S-transferase (GST), and total antioxidant capacity (T-AOC) indicated increasing risks of reproductive dysfunction by Pb. Furthermore, increasing apoptosis and upregulation of gene expression associated with cell death suggested testicular abnormal development. In addition, molecular signaling involved with steroidogenesis in the testis was disturbed by Pb treatment. The study showed that Pb could impair testicular development and reproductive function by morphological and histological injury, hormone suppression, oxidative stress, cell death, and HPG axis disruption.







Similar content being viewed by others
Data Availability
Data are available from the corresponding author on reasonable request.
Abbreviations
- AR:
-
Androgen receptors
- AST:
-
Cross-sectional area of the seminiferous tubules
- Bcl-2:
-
B-cell lymphoma-2
- CAT:
-
Catalase
- Casp-3:
-
Caspase-3
- Casp-9:
-
Caspase-9
- Cyt-c:
-
Cytochrome c
- DAX-1:
-
Dosage-sensitive sex reversal adrenal hypoplasia congenita critical region on the X chromosome, gene 1
- FSH:
-
Follicle stimulating hormone
- FSHR:
-
Follicle-stimulating hormone receptor
- FCR:
-
Feed conversion ratio
- GSH:
-
Glutathione
- GST:
-
Glutathione-S-transferase
- HPG axis:
-
Hypothalamus-pituitary–gonadal axis
- GnRH:
-
Gonadotropin-releasing hormone
- IL-1β:
-
Interleukin 1 beta
- LH:
-
Luteinizing hormone
- LHR:
-
Luteinizing hormone receptor
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor kappa B
- TNF-α:
-
Tumor necrosis factor alpha
- P450scc:
-
Cytochrome P450 side-chain cleavage enzyme
- P450c17:
-
Cytochrome P450 17α-hydroxylase/17,20-lyase
- ROS:
-
Reactive oxygen species
- SET:
-
Seminiferous epithelial thickness
- SF-1:
-
Steroidogenic factor 1
- SLD:
-
Seminiferous luminal diameter
- SOD:
-
Superoxide dismutase
- StAR:
-
Steroidogenic acute regulatory protein
- STD:
-
Seminiferous tubular diameter
- T:
-
Testosterone
- T-AOC:
-
Total antioxidant capacity
- 3β-HSD:
-
3β-Hydroxysteroid dehydrogenase
- 17β-HSD:
-
17β-Hydroxysteroid dehydrogenase
References
Sindhu KK, Sutherling WW (2015) Role of lead in the central nervous system: effect on electroencephalography, evoked potentials, electroretinography, and nerve conduction. Neurodiagn J 55(2):107–121. https://doi.org/10.1080/21646821.2015.1043222
Gandhi J, Hernandez RJ, Chen A, Smith NL, Sheynkin YR, Joshi G, Khan SA (2017) Impaired hypothalamic-pituitary-testicular axis activity, spermatogenesis, and sperm function promote infertility in males with lead poisoning. Zygote 25(2):103–110. https://doi.org/10.1017/S0967199417000028
He L, Chen Z, Dai B, Li G, Zhu G (2018) Low-level lead exposure and cardiovascular disease: the roles of telomere shortening and lipid disturbance. J Toxicol Sci 43(11):623–630. https://doi.org/10.2131/jts.43.623
Wang L, Zheng Y, Zhang G, Han X, Li S, Zhao H (2021) Lead exposure induced inflammation in bursa of Fabricius of Japanese quail (C. japonica) via NF-κB pathway activation and Wnt/β-catenin signaling inhibition. J Inorg Biochem 224: 111587. https://doi.org/10.1016/j.jinorgbio.2021.111587
Chen J, Tong Y, Xu J, Liu X, Li Y, Tan M, Li Y (2012) Environmental lead pollution threatens the children living in the Pearl River Delta region. China Environ Sci Pollut Res Int 19(8):3268–3275. https://doi.org/10.1007/s11356-012-0837-9
Finkelstein ME, Doak DF, George D, Burnett J, Brandt J, Church M, Grantham J, Smith DR (2012) Lead poisoning and the deceptive recovery of the critically endangered California condor. Proc Natl Acad Sci U S A 109(28):11449–11454. https://doi.org/10.1073/pnas.1203141109
Grade TJ, Pokras MA, Laflamme EM, Vogel HS (2018) Population-level effects of lead fishing tackle on common loons. J Wildl Manage 82:155–164. https://doi.org/10.1002/jwmg.21348
Pain DJ, Mateo R, Green RE (2019) Effects of lead from ammunition on birds and other wildlife: a review and update. Ambio 48(9):935–953. https://doi.org/10.1007/s13280-019-01159-0
Kou H, Fu Y, He Y, Jiang J, Gao X, Zhao H (2019) Chronic lead exposure induces histopathological damage, microbiota dysbiosis and immune disorder in the cecum of female Japanese quails (Coturnix japonica). Ecotoxicol Environ Saf 183:109588. https://doi.org/10.1016/j.ecoenv.2019.109588
Kou H, Ya J, Gao X, Zhao H (2020) The effects of chronic lead exposure on the liver of female Japanese quail (Coturnix japonica): histopathological damages, oxidative stress and AMP-activated protein kinase based lipid metabolism disorder. Ecotoxicol Environ Saf 190:110055. https://doi.org/10.1016/j.ecoenv.2019.110055
Fritsch C, Jankowiak Ł, Wysocki D (2019) Exposure to Pb impairs breeding success and is associated with longer lifespan in urban European blackbirds. Sci Rep 9(1):486. https://doi.org/10.1038/s41598-018-36463-4
He Y, Wang L, Li X, Zhao H (2020) The effects of chronic lead exposure on the ovaries of female juvenile Japanese quails (Coturnix japonica): developmental delay, histopathological alterations, hormone release disruption and gene expression disorder. Ecotoxicol Environ Saf 205:111338. https://doi.org/10.1016/j.ecoenv.2020.111338
Aithamadouche N, Sadi N, Kharoubi O, Slimani M, Aoues A (2013) The protective effect of vitamin E against genotoxicity of lead acetate intraperitoneal administration in male rat. Not Sci Biol 5(4):412–419. https://doi.org/10.15835/nsb549125
Mabrouk A (2018) Therapeutic effect of thymoquinone against lead-induced testicular histological damage in male Wistar rats. Andrologia 50(6):e13014. https://doi.org/10.1111/and.13014
Huang H, Wang Y, An Y, Jiao W, Xu Y, Han Q, Teng X, Teng X (2019) Selenium alleviates oxidative stress and autophagy in lead-treated chicken testes. Theriogenology 131:146–152. https://doi.org/10.1016/j.theriogenology.2019.03.015
Vallverdú-Coll N, Mougeot F, Ortiz-Santaliestra ME, Castaño C, Santiago-Moreno J, Mateo R (2016) Effects of lead exposure on sperm quality and reproductive success in an avian model. Environ Sci Technol 50(22):12484–12492. https://doi.org/10.1021/acs.est.6b04231
Vigeh M, Smith DR, Hsu PC (2011) How does lead induce male infertility? Iran J Reprod Med 9(1):1–8
Matuq Al-Yasi H, El-Shazly SA, Ahmed EF, Hasan Alamer K, Hessini KY, Attia HA, Alkafafy ME, Mohamed AA, Hassan FA (2021) Protective effects of taif rosewater against testicular impairment induced by lead intoxication in rats. Andrologia 53(6):e14045. https://doi.org/10.1111/and.14045
Huss D, Poynter G, Lansford R (2008) Japanese quail (Coturnix japonica) as a laboratory animal model. Lab Anim 37(11):513–519. https://doi.org/10.1038/laban1108-513
Morgan GW, Edens FW, Thaxton P, Parkhurst CR (1975) Toxicity of dietary lead in Japanese quail. Poult Sci 54(5):1636–1646. https://doi.org/10.3382/ps.0541636
Stone CL, Soares JH Jr (1976) The effect of dietary selenium level on lead toxicity in the Japanese quail. Poult Sci 55(1):341–349. https://doi.org/10.3382/ps.0550341
Banerjee S, Shahin S, Chaturvedi CM (2018) Age dependent variations in the deep brain photoreceptors (DBPs), GnRH-GnIH system and testicular steroidogenesis in Japanese quail, Coturnix coturnix japonica. Exp Gerontol 108:7–17. https://doi.org/10.1016/j.exger.2018.03.018
Kyrylkova K, Kyryachenko S, Leid M, Kioussi C (2012) Detection of apoptosis by TUNEL assay. Methods Mol Biol 887:41–47. https://doi.org/10.1007/978-1-61779-860-3_5
Ahmad F, Liu P (2020) (Ascorb)ing Pb Neurotoxicity in the developing brain. Antioxidants (Basel) 9(12):1311. https://doi.org/10.3390/antiox9121311
White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR (2007) New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol 225(1):1–27. https://doi.org/10.1016/j.taap.2007.08.001
Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158. https://doi.org/10.1016/j.neuro.2016.07.013
Santa Maria MP, Hill BD, Kline J (2019) Lead (Pb) neurotoxicology and cognition. Appl Neuropsychol Child 8(3):272–293. https://doi.org/10.1080/21622965.2018.1428803
Sokol RZ (1987) Hormonal effects of lead acetate in the male rat: mechanism of action. Biol Reprod 37(5):1135–1138. https://doi.org/10.1095/biolreprod37.5.1135
Kruk MR, Westphal KG, Van Erp AM, van Asperen J, Cave BJ, Slater E, de Koning J, Haller J (1998) The hypothalamus: cross-roads of endocrine and behavioural regulation in grooming and aggression. Neurosci Biobehav Rev 23(2):163–177. https://doi.org/10.1016/s0149-7634(98)00018-9
Leng G, MacGregor DJ (2018) Models in neuroendocrinology. Math Biosci 305:29–41. https://doi.org/10.1016/j.mbs.2018.07.008
Moore AM, Coolen LM, Lehman MN (2022) In vivo imaging of the GnRH pulse generator reveals a temporal order of neuronal activation and synchronization during each pulse. Proc Natl Acad Sci U S A 119(6):e2117767119. https://doi.org/10.1073/pnas.2117767119
Osada M, Treen N (2013) Molluscan GnRH associated with reproduction. Gen Comp Endocrinol 181:254–258. https://doi.org/10.1016/j.ygcen.2012.09.002
Luo J, Yang Y, Zhang T, Su Z, Yu D, Lin Q, Chen H, Zhang Q, Xiang Q, Xue W, Ge R, Huang Y (2018) Nasal delivery of nerve growth factor rescue hypogonadism by up-regulating GnRH and testosterone in aging male mice. EBio Medicine 35:295–306. https://doi.org/10.1016/j.ebiom.2018.08.021
Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N (2008) Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol 109(3–5):323–330. https://doi.org/10.1016/j.jsbmb.2008.03.004
Ooi GT, Tawadros N, Escalona RM (2004) Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol 228(1–2):1–21. https://doi.org/10.1016/j.mce.2004.07.018
Ait Hamadouche N, Nesrine S, Abdelkeder A (2013) Lead toxicity and the hypothalamic-pituitary-testicular axis. Not Sci Biol 5(1): 1–6. https://doi.org/10.15835/nsb518038
Sambroni E, Rolland AD, Lareyre JJ, Le Gac F (2012) FSH and LH have common and distinct effects on gene expression in rainbow trout testis. J Mol Endocrinol 50(1):1–18. https://doi.org/10.1530/JME-12-0197
Ye L, Su ZJ, Ge RS (2011) Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules 16(12):9983–10001. https://doi.org/10.3390/molecules16129983
Gao HT, Di QN, Qian LL, Lu L, Li RX, Cao WX, Xu Q (2020) Zinc supplement ameliorates phthalates-induced reproductive toxicity in male rats. Chemosphere 246:125828. https://doi.org/10.1016/j.chemosphere.2020.125828
Zirkin BR, Papadopoulos V (2018) Leydig cells: formation, function, and regulation. Biol Reprod 99(1):101–111. https://doi.org/10.1093/biolre/ioy059
Sorwell KG, Urbanski HF (2010) Dehydroepiandrosterone and age-related cognitive decline Age (Dordr) 32(1):61–67. https://doi.org/10.1007/s11357-009-9113-4
Li X, Guo J, Jiang X, Sun J, Tian L, Jiao R, Tang Y, Bai W (2019) Cyanidin-3-O-glucoside protects against cadmium-induced dysfunction of sex hormone secretion via the regulation of hypothalamus-pituitary-gonadal axis in male pubertal mice. Food Chem Toxicol 129:13–21. https://doi.org/10.1016/j.fct.2019.04.033
Klemm RD, Knight CE, Stein S (1973) Gross and microscopic morphology of the glandula proctodealis (foam gland) of Coturnix c. japonica (aves). J Morphol 141(2): 171–184. https://doi.org/10.1002/jmor.1051410205
Singh RP, Sastry KV, Pandey NK, Singh KB, Malecki IA, Farooq U, Mohan J, Saxena VK, Moudgal RP (2012) The role of the male cloacal gland in reproductive success in Japanese quail (Coturnix japonica). Reprod Fertil Dev 24(2):405–409. https://doi.org/10.1071/RD11057
Lin L, Achermann JC (2008) Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev 2(4–5):200–209. https://doi.org/10.1159/000152036
Młynarczuk J, Rękawiecki R (2010) The role of the orphan receptor SF-1 in the development and function of the ovary. Reprod Biol 10(3):177–193
Gardiner JR, Shima Y, Morohashi K, Swain A (2012) SF-1 expression during adrenal development and tumourigenesis. Mol Cell Endocrinol 351(1):12–18. https://doi.org/10.1016/j.mce.2011.10.007
Martin LJ, Boucher N, El-Asmar B, Tremblay JJ (2009) cAMP-induced expression of the orphan nuclear receptor Nur77 in MA-10 Leydig cells involves a CaMKI pathway. J Androl 30(2):134–145. https://doi.org/10.2164/jandrol.108.006387
Qamar I, Gong EY, Kim Y, Song CH, Lee HJ, Chun SY, Lee K (2010) Anti-steroidogenic factor ARR19 inhibits testicular steroidogenesis through the suppression of Nur77 transactivation. J Biol Chem 285(29):22360–22369. https://doi.org/10.1074/jbc.M109.059949
Guo IC, Hu MC, Chung BC (2003) Transcriptional regulation of CYP11A1. J Biomed Sci 10(6 Pt 1):593–598. https://doi.org/10.1159/000073524
Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, Mellon SH, Lee K (2004) Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol 24(7):2593–2604. https://doi.org/10.1128/MCB.24.7.2593-2604.2004
Wang ZJ, Jeffs B, Ito M, Achermann JC, Yu RN, Hales DB, Jameson JL (2001) Aromatase (Cyp19) expression is up-regulated by targeted disruption of Dax1. Proc Natl Acad Sci U S A 98(14):7988–7993. https://doi.org/10.1073/pnas.141543298
Song KH, Park YY, Park KC, Hong CY, Park JH, Shong M, Lee K, Choi HS (2004) The atypical orphan nuclear receptor DAX-1 interacts with orphan nuclear receptor Nur77 and represses its transactivation. Mol Endocrinol 18(8):1929–1940. https://doi.org/10.1210/me.2004-0043
Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15(6):1583–1606. https://doi.org/10.1089/ars.2011.3999
Li X, Zheng Y, Zhang G, Wang R, Jiang J, Zhao H (2021) Cadmium induced cardiac toxicology in developing Japanese quail (Coturnix japonica): histopathological damages, oxidative stress and myocardial muscle fiber formation disorder. Comp Biochem Physiol C Toxicol Pharmacol 250:109168. https://doi.org/10.1016/j.cbpc.2021.109168
Carvalho Cdos S, Bernusso VA, Fernandes MN (2015) Copper levels and changes in pH induce oxidative stress in the tissue of curimbata (Prochilodus lineatus). Aquat Toxicol 167:220–227. https://doi.org/10.1016/j.aquatox.2015.08.003
Lee JW, Choi H, Hwang UK, Kang JC, Kang YJ, Kim KI, Kim JH (2019) Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: a review. Environ Toxicol Pharmacol 68:101–108. https://doi.org/10.1016/j.etap.2019.03.010
Koivula MJ, Eeva T (2010) Metal-related oxidative stress in birds. Environ Pollut 158(7):2359–2370. https://doi.org/10.1016/j.envpol.2010.03.013
Devóz PP, Reis MBD, Gomes WR, Maraslis FT, Ribeiro DL, Antunes LMG, Batista BL, Grotto D, Reis RM, Barbosa F Jr, Barcelos GRM (2021) Adaptive epigenetic response of glutathione (GSH)-related genes against lead (Pb)-induced toxicity, in individuals chronically exposed to the metal. Chemosphere 269:128758. https://doi.org/10.1016/j.chemosphere.2020.128758
Jin Y, Wang L, Ruan M, Liu J, Yang Y, Zhou C, Xu B, Fu Z (2011) Cypermethrin exposure during puberty induces oxidative stress and endocrine disruption in male mice. Chemosphere 84(1):124–130. https://doi.org/10.1016/j.chemosphere.2011.02.034
Sikka SC (2001) Relative impact of oxidative stress on male reproductive function. Curr Med Chem 8(7):851–862. https://doi.org/10.2174/0929867013373039
Rezaei N, Mardanshahi T, Shafaroudi MM, Abedian S, Mohammadi H, Zare Z (2018) Effects of l-carnitine on the follicle-stimulating hormone, luteinizing hormone, testosterone, and testicular tissue oxidative stress levels in streptozotocin-induced diabetic rats. J Evid Based Integr Med 23: 2515690X18796053. https://doi.org/10.1177/2515690X18796053
Abdel-Emam RA, Ahmed EA (2021) Ameliorative effect of L-carnitine on chronic lead-induced reproductive toxicity in male rats. Vet Med Sci 7(4):1426–1435. https://doi.org/10.1002/vms3.473
Çeribaşı S, Türk G, Özçelik M, Doğan G, Çeribaşı AO, Mutlu Sİ, Erişir Z, Güvenç M, Güngören G, Acısu TC, Akarsu SA, Kaya ŞÖ, Sönmez M, Yüce A, Çiftçi M, Çambay Z, Bağcı E, Azman MA, Şimşek ÜG (2020) Negative effect of feeding with high energy diets on testes and metabolic blood parameters of male Japanese quails, and positive role of milk thistle seed. Theriogenology 144:74–81. https://doi.org/10.1016/j.theriogenology.2019.12.021
Vitagliano O, Addeo R, D’Angelo V, Indolfi C, Indolfi P, Casale F (2013) The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev Hematol 6(5):587–597. https://doi.org/10.1586/17474086.2013.827415
Wang Q, Zhang L, Yuan X, Ou Y, Zhu X, Cheng Z, Zhang P, Wu X, Meng Y, Zhang L (2016) The relationship between the Bcl-2/Bax proteins and the mitochondria-mediated apoptosis pathway in the differentiation of adipose-derived stromal cells into neurons. PLoS One 11(10):e0163327. https://doi.org/10.1371/journal.pone.0163327
Zhang T, Yan Z, Zheng X, Wang S, Fan J, Liu Z (2020) Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (Corbicula fluminea). Fish Shellfish Immunol 99:514–525. https://doi.org/10.1016/j.fsi.2020.02.046
Winnall WR, Muir JA, Hedger MP (2011) Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J Leukoc Biol 90(1):133–143. https://doi.org/10.1189/jlb.1010557
Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R (2012) Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod Toxicol 34(3):298–307. https://doi.org/10.1016/j.reprotox.2012.06.007
Hu X, Chi Q, Wang D, Chi X, Teng X, Li S (2018) Hydrogen sulfide inhalation-induced immune damage is involved in oxidative stress, inflammation, apoptosis and the Th1/Th2 imbalance in broiler bursa of Fabricius. Ecotoxicol Environ Saf 164:201–209. https://doi.org/10.1016/j.ecoenv.2018.08.029
Lysiak JJ (2004) The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol 2:9. https://doi.org/10.1186/1477-7827-2-9
Acknowledgements
We are grateful for Ms. Yuhan Fang for experimental assistance and for Ms. Xuan Li, Ling Wang, and Gaixia Zhang for valuable suggestions about the manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (No. 33372201).
Author information
Authors and Affiliations
Contributions
Zheng Y: Conceptualization, Methodology, Data curation, Writing-Original draft preparation; Zhang QY, Jing LY, and Fei YF: Data curation, Visualization, and Software; Zhao HF: Conceptualization, Methodology, Writing-Original draft preparation, Supervision; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Animal care and experiment operations were approved and conducted in accordance with the Animal Care Guidelines of Shaanxi Normal University (No. SNNU52).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, Y., Zhang, Q., Jing, L. et al. The Effects of Chronic Lead Exposure on Testicular Development of Japanese Quail (Coturnix japonica): Histopathological Damages, Oxidative Stress, Steroidogenesis Disturbance, and Hypothalamus-Pituitary-Testis Axis Disruption. Biol Trace Elem Res 201, 3446–3460 (2023). https://doi.org/10.1007/s12011-022-03436-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03436-8