Abstract
Medullary Thyroid Carcinoma (MTC) constitutes around 5% of all thyroid cancers. Trace elements assessment has emerged as a useful strategy in the diagnostics of MTC combined with Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of Matrix Metalloproteinases (TIMPs) analysis. The aim of this study was to compare the presence and content of trace elements (i.e., Copper (Cu), Zinc (Zn), Iron (Fe), and Manganese (Mn)) in MTC with respect to control samples and their potential relationship with markers of MTC in tissues. The study included 26 patients who had undergone thyroidectomy, due to the diagnosis of MTC and 17 patients as control. We combined tumour pathology and staging, immunohistochemical analysis of calcitonin, MMPs, and TIMPs, with analytical biochemistry using Inductively Coupled Plasma – Mass Spectrometry (ICP-MS) to determine the levels of trace elements. No differences by MTC type for MMPs and their TIPMs, although strong TIMP-1 and TIMP-2 immunohistochemical expression of MTC were unveiled. Additionally, Zn, Fe, and Mn tended to be decreased, and Cu to be increased in samples presenting MTC with respect to controls. Moreover, Zn was the unique trace element which seemed to be correlated with MMPs and TIMPs. Trace elements such as Zn, Fe, and Mn are decreased in tissues affected by MTC. In addition, Zn may be the trace element which saves more relationship with the proportion and intensity of MMPs, being considered altogether useful biomarkers of MTC. We therefore suggest the analysis of novel and traditional markers of MTC as a novel approach in this pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medullary Thyroid Carcinoma (MTC) is a form of thyroid carcinoma originated in the parafollicular cells, where calcitonin hormone – a sensitive biomarker used for its diagnosis – is produced. MTC, which can be both sporadic and hereditary, constitutes around 5% of all thyroid cancers, being in the third place of most frequent thyroid carcinomas [1,2,3,4,5].
MTC is characterized by the presence of high levels of calcitonin and the Zinc (Zn) peptidase–Matrix Metalloproteinases 2 and 9 (MMP-2 and MMP-9) and their Tissue Inhibitors of Matrix Metalloproteinases (TIMPs) [6, 7]. MMPs are overexpressed in a variety of malignant tumours and are a unique family of Zn-binding endopeptidases that can degrade the components of the extracellular matrix, which is essential in invasive growth and metastasizing processes [8]. In this regard, MMPs have a different role in tumour progression depending on the enzymatic activity, the type of enzyme producing cells, and the tumour stages [9]. Additionally, expression of TIMPs, which are proxies of tumour progression, are associated with angiogenesis, invasion, and metastasis in carcinogenic processes [10].
Trace elements assessment has emerged as a useful strategy in the diagnostics of MTC combined with MMPs and their TIMPs analysis. Zn plays a substantial role in (i) cell proliferation, (ii) the regulation of chronic inflammatory status reducing inflammatory cytokines, (iii) the reduction of oxidative stress via synthesizing antioxidant enzymes, and (iv) catalysing multiple enzymes [11]. A recent study suggested that the status of certain trace elements such as Zn are important in MTC pathophysiology – their levels being increased and a potent risk factor for this type of carcinoma – [12]. The relationship between Zn and thyroid metabolism is based on the hypothesis that the nuclear T3 receptor contains Zn binding protein [13].
However, although the role of Zn upon MTC has been widely studied, the role of other trace elements (e.g., Copper (Cu), Iron (Fe) and Manganese (Mn)) on MTC has not been previously elucidated or has been studied in other types of thyroid carcinomas (i.e., papillary thyroid carcinoma (PTC)) [14]. Therefore, the aim of this study was to compare the presence and content of trace elements (i.e., Cu, Zn, Fe, and Mn) in MTC with respect to control samples and their potential relationship with markers of MTC in tissues.
Materials and Methods
Study Participants
The present study included 26 MTC samples (73% women) aged 21 to 77 years who had undergone thyroidectomy at the Centre for Endocrine Surgery of the Institute of Endocrinology, Diabetes and Metabolic Diseases, Clinical Centre of Serbia, Belgrade, during 2012 and 2013. Seventeen samples were included in the study as controls – their tissue samples being obtained from patients treated for benign nodular hyperplasia –.
This study abides by the Declaration of Helsinki on research involving human subjects and was approved by the Ethics Committee of the Faculty of Medicine, University of Belgrade No. 29/XI-10 for studies involving human subjects. The samples’ identity remained anonymous. Written informed consent was obtained from all individual participants after explaining goals of the study.
Classification according to International Union Against Cancer was applied to classify tumours. Groups were formed by the following criteria: T category 1 (≤ 20 mm) and T category 2 (20–40 mm) [15].
Samples Treatment and Measurement
Calcitonin in Serum Analysis
Calcitonin in serum was determined by chemiluminescent immunoassays on the automatic Analyzer IMMULITE 100 Siemens (Germany).
Tissue Microarrays (TMA)
Samples were fixed with 10% formalin and embedded in paraffin blocks. A high-density TMA was constructed manually. Previously marked area of interest on slides was translated to corresponding regions of donor paraffin blocks. Needle with inner diameter of 1.1 mm was used to create and transfer tissue cores (0.785 mm2 crosscut surface area) in recipient paraffin blocks. Two cores were taken from every lesion. Cases with at least one section across all slides were regarded as valid. Tissue cores with external controls were included in all TMAs. Final TMA block consisted of 60 cores (10 × 6), plus two control tissue cores. Control tissues included in TMA were normal thyroid tissues [16,17,18,19].
Immunohistochemistry
Immunohistochemical staining of Calcitonin in Tissues (CT) (DAKO, pAb, RTU), MMP-9 (Sigma-Aldrich, pAb, 1:300), MMP-2 (Sigma-Aldrich, pAb, 1:50), TIMP-1 (Abcam, Clone 102D1, 1:100), and TIMP-2 (Abcam, Clone 3A4, 1:100) was done manually according to the manufacturer instructions as shown in Supplementary Table 1.
Evaluation of Immunohistochemical Staining
Cytoplasmatic ± membranous immunoreactivity for CT, MMP-9, MMP-2, TIMP-1, and TIMP-2 in more than 10% of cells was considered as positive staining without regard to intensity of staining. In respect to percentage of positive cells (P) of staining, we graded staining as 0, 1, 2, 3, when 0–10%, 11–25%, 26–50%, 51–100% of tumours cells showed expression, respectively. We also scored the overall intensity (I) such as 0, 1, 2, 3 for no staining at all, weak, moderate, and intensive staining, respectively. All the previous immunohistochemical procedures were followed according to standard protocols [20].
Trace Elements Analysis
A quadrupole Inductively Coupled Plasma – Mass Spectrometry (ICP-MS) system (model Agilent 7500ce from Agilent Technologies, Tokyo, Japan) was used for total multi-element quantification. Torch position and ion lens voltage settings of ICP-MS were optimized daily for optimum sensitivity and control of oxide formation with a multi-element standard solution (1 ng/mL−1 Li, Co, Y, Ce and Tl, in 1% w/w HNO3). The following trace elements were monitored by ICP-MS 7500ce: Fe, Mn, Cu, and Zn. The quadrupole ICP-MS was equipped with an octupole collision cell to remove polyatomic interferences for selected analytes, operating the collision cell with a H2 gas flow of 4.0 mL·min−1. Optimized operating conditions are shown in Supplementary Table 2.
Quantitative Multi-element Analysis of Zn, Cu, Fe and Mn in Paraffin Embedded MTC and Control Samples
Total quantification of essential trace elements (i.e., Zn, Fe, Cu and Mn) was carried out by conventional nebulization ICP-MS after acidic digestion of the 26 paraffin-embedded tissues which were mineralized. Briefly, samples weighing from 10 to 30 mg were digested using 750 µL of concentrated high purity grade HNO3 (65% v/v, Suprapur® Merck, Germany) and heated 2 h at 90 °C, under high pressure. Preparatory blank tubes containing paraffin (no tissue added) were similarly treated as samples and used as background controls. Mineralized solutions were diluted with Milli-Q water to reach 2% of HNO3, using Ga (10 ng·mL−1) as internal standard. External calibration curve of Zn, Cu, Fe, and Mn were performed by the preparation of Zn, Cu, Fe and Mn standard solutions (0–250 ng·mL−1) in 2% HNO3 using Ga (10 ng·mL−1) as internal standard. Control samples were prepared following the same procedure.
Statistical Analysis
As a previous step to the execution of a parametric model or not, the hypothesis of normal distribution was rejected using the Shapiro–Wilk test, visual check of histograms, Q-Q, and box plots. Descriptive statistics have been used for data expression of age, calcitonin in serum, Cu, Zn, Fe, and Mn, indicating the results of the numerical variables such as Median (M) and Interquartile Range (IQR). Frequency statistics was used for data expression of category of tumour, sex, MMPs, TIMPs, and CT, indicating the results of the variables as frequency (N) and percentage (%). Mann–Whitney test was used to compare the median distribution of calcitonin in serum and trace elements by median age. Chi square test was used for correlating categorical variables, i.e., the relationship between immunohistochemistry of MTC markers and the category of tumour. Linear correlation analysis was used to estimate the degree of association between trace elements and MMPs and their TIMPs using Spearman’s correlation coefficient (Rho). Statistical significance was defined as p value < 0.05. The statistical analysis was performed using the SPSS 22.0 statistical program for MAC (SPSS Inc. Chicago, IL, USA). For the graphical plots, GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA) was used.
Results
The general characteristics of the samples of this study are represented in Table 1. One-third of the samples presented T2 MTC. Moreover, females represented more than two-thirds of the samples with MTC. Calcitonin in serum, Cu, Zn, Fe, and Mn, showed no median differences by age (all P > 0.05).
The immunohistochemical expression of MMPs and their TIMPs are represented in Fig. 1. The results of our study showed weak-moderate expression MMP-2 and MMP-9 but strong TIMP-1 and TIMP-2 immunohistochemical expression of MTC.
Immunohistochemistry of MTC markers by T category, is found in Table 2. No significant changes were observed for the analyzed parameters by T category of MTC (all P ≥ 0.088).
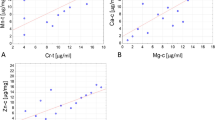
Figure 2 shows the median differences of trace elements values in MTC group vs control group. Cu was increased in MTC group with respect to control group (P ≤ 0.001; Panel A), whereas Zn, Fe and Mn were decreased in MTC group when compared to control group (all P ≤ 0.001; Panels B–D).
Table 3 shows the Spearman’s correlation analysis between trace elements and MMPs, their TIMPs and CT. Zn correlated inversely with I-MMP-2, P-MMP-9, I-MMP-9, and P-CT (all P ≤ 0.048), whereas no relationship was observed with the rest of MMPs, their TIMPs and CT (All P ≥ 0.119). Cu, Fe, and Mn showed no relationship with MMPs, their TIMPs and CT (all P ≥ 0.059).
Discussion
The present results showed no differences by MTC type for MMPs and their TIPMs, although strong TIMP-1 and TIMP-2 immunohistochemical expression of MTC was unveiled. Additionally, trace elements such as Zn, Fe, and Mn were observed to be decreased, and Cu to be increased in samples presenting MTC with respect to controls. Moreover, Zn was the unique trace element which seemed to be correlated with MMPs. These findings shed light to the idea that trace elements combined with MMPs and their TIMPs analysis could be useful markers of MTC, with Zn being the trace element of reference for this pathology.
The results of our study showed weak-moderate MMP-2, MMP-9 and strong TIMP-2, TIMP-1 immunohistochemical expression of MTC, although no differences by MTC type were observed. During cancer progression, high levels of TIMPs are associated with the inhibition of tumour growth, angiogenesis, invasion and metastasis, secondary to the inhibition of endothelial cell migration [21, 22]. Expression of MMP-9 and TIMP-2 in MTC have been analysed by other authors as well. In this regard, one study reported weak MMP and TIMP immunostaining in MTC [23]. Additionally, increased TIMP-2 levels have been suggested as a marker of low metastatic potential in MTC, since it was observed to be inversely correlated to calcitonin and tumor stage [22].
In the recent years, some studies have shown the important role of trace elements in different types of diseases including malignant tissues [24, 25] and many of the studies showed lower concentrations of Zn content in malignant tissues compared to healthy control samples [26, 27]. In line with these results, we showed a statistically significant decrease in Zn, Fe and Mn concentrations in the MTC group compared to the control group. Additionally, we demonstrated a significant increase in Cu ions concentrations in the group of samples with MTC compared to the controls. Interestingly, similar results were found in a study examining the importance of Cu concentrations in other types of thyroid carcinoma, showing a statistically significant increase in Cu concentrations of samples with thyroid carcinoma compared to the samples with Benign Thyroid Disease (BTD) [7]. A potential explanation for higher Cu concentrations in patients with thyroid carcinoma may be a possible dysregulation in metabolism of amino acids included in Cu chelating. As integral part of antioxidant enzyme Superoxide Dismutase (SOD), Zn and Cu levels could also be analysed in the context of SOD activity. Oxidative tissue damage during carcinogenesis may reduce the activity of SOD, which in turn could affect both tissue concentrations of Cu and Zn [28, 29]; however, regretfully, we did not measure SOD.
In our study, Cu, Fe, and Mn showed no relationship with MMPs, their TIMPs and CT, whereas Zn was inversely related with the MMPs of the study, but not with their TIMPs. Similarly to our results, situations of hypozincemia have been reported to be directly related to positive expression of MMPs 2 and 9, but in non-tumorous samples [30]. Moreover, although TIMPs are usually designed to interact with Zn ions in the catalytic domain, thus interfering with enzymatic activity [31], Zn did not correlate with TIMPs in our samples. Studies performed in mice, showed that in case of Zn deficiency, the expression of TIMPs was increased but the expression of MMPs was decreased [32]. Therefore, the role of Zn upon MMPs and their TIMPs may be controversial in some cases.
The present study had some limitations and strengths. As strengths, this study is one of the few of its kind published to date combining MMP-2, MMP-9 and their TIMPs in tissues with trace elements analysis. In this regard, this study emphasizes more than previous publications that assessing altogether the previously mentioned parameters could be an effective strategy for the diagnostic of MTC as useful biomarkers. As limitations, this cross-sectional study enrolled lower samples than desired. This was limited to the difficulty of finding samples presenting diagnosis of MTC who voluntary wanted to take part of the study. Moreover, the cross-sectional nature of the study was a limitation for establishing causality to the correlations observed between the main parameters of our study.
Conclusions
The findings of the present study suggest a high tendency towards decreased levels of trace elements such as Zn, Fe, and Mn in tissues affected by MTC. In addition, Zn may be the trace element which saves more relationship with the proportion and intensity of MMPs, being altogether useful biomarkers of MTC. We therefore suggest the analysis of novel and traditional markers of MTC as a novel approach in this pathology.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to their confidentiality but are available from the corresponding author on reasonable request.
References
Kebebew E, Greenspan FS, Clark OH et al (2005) Extent of disease and practice patterns for medullary thyroid cancer. J Am Coll Surg 200:890–896. https://doi.org/10.1016/j.jamcollsurg.2004.12.011
Wells SA, Asa SL, Dralle H et al (2015) Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid Off J Am Thyroid Assoc 25:567–610. https://doi.org/10.1089/thy.2014.0335
Costante G, Durante C, Francis Z et al (2009) Determination of calcitonin levels in C-cell disease: clinical interest and potential pitfalls. Nat Clin Pract Endocrinol Metab 5:35–44. https://doi.org/10.1038/ncpendmet1023
Chambon G, Alovisetti C, Idoux-Louche C et al (2011) The use of preoperative routine measurement of basal serum thyrocalcitonin in candidates for thyroidectomy due to nodular thyroid disorders: results from 2733 consecutive patients. J Clin Endocrinol Metab 96:75–81. https://doi.org/10.1210/jc.2010-0162
Ahmed SR, Ball DW (2011) Clinical review: incidentally discovered medullary thyroid cancer: diagnostic strategies and treatment. J Clin Endocrinol Metab 96:1237–1245. https://doi.org/10.1210/jc.2010-2359
Cavalheiro BG, Junqueira CR, Brandão LG (2008) Expression of matrix metalloproteinase 2 (MMP-2) and tissue inhibitor of metalloproteinase 2 (TIMP-2) in medullary thyroid carcinoma: prognostic implications. Thyroid Off J Am Thyroid Assoc 18:865–871. https://doi.org/10.1089/thy.2007.0412
Dragutinović VV, Tatić SB, Nikolić-Mandić SD et al (2014) Copper as ancillary diagnostic tool in preoperative evaluation of possible papillary thyroid carcinoma in patients with benign thyroid disease. Biol Trace Elem Res 160:311–315. https://doi.org/10.1007/s12011-014-0071-z
Liotta LA, Tryggvason K, Garbisa S et al (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284:67–68. https://doi.org/10.1038/284067a0
McCawley LJ, Matrisian LM (2000) Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today 6:149–156. https://doi.org/10.1016/s1357-4310(00)01686-5
Olechnowicz J, Tinkov A, Skalny A, Suliburska J (2018) Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 68:19–31. https://doi.org/10.1007/s12576-017-0571-7
Bonaventura P, Benedetti G, Albarède F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14:277–285. https://doi.org/10.1016/j.autrev.2014.11.008
Emami A, Nazem MR, Shekarriz R, Hedayati M (2017) Micronutrient status (calcium, zinc, vitamins D and E) in patients with medullary thyroid carcinoma: a cross-sectional study. Nutr Burbank Los Angel Cty Calif 41:86–89. https://doi.org/10.1016/j.nut.2017.04.004
Freake HC, Govoni KE, Guda K et al (2001) Actions and interactions of thyroid hormone and zinc status in growing rats. J Nutr 131:1135–1141. https://doi.org/10.1093/jn/131.4.1135
Pellegriti G, De Vathaire F, Scollo C et al (2009) Papillary thyroid cancer incidence in the volcanic area of Sicily. J Natl Cancer Inst 101:1575–1583. https://doi.org/10.1093/jnci/djp354
Tuttle RM, Haugen B, Perrier ND (2017) Updated American Joint Committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (Eighth Edition): what changed and why? Thyroid Off J Am Thyroid Assoc 27:751–756. https://doi.org/10.1089/thy.2017.0102
Eckel-Passow JE, Lohse CM, Sheinin Y et al (2010) Tissue microarrays: one size does not fit all. Diagn Pathol 5:48. https://doi.org/10.1186/1746-1596-5-48
Wang S-L, Yang C-H, Chen H-H, Chai C-Y (2006) A simple and economical method for the manual construction of well-aligned tissue arrays. Pathol - Res Pract 202:485–486. https://doi.org/10.1016/j.prp.2006.01.014
Singh DK, Sakhuja P, Gondal R (2009) Making and using inexpensive manually constructed tissue micro-array: experience of a tertiary care hospital in India. Indian J Pathol Microbiol 52:304–309. https://doi.org/10.4103/0377-4929.54980
Hassan S, Ferrario C, Mamo A, Basik M (2008) Tissue microarrays: emerging standard for biomarker validation. Curr Opin Biotechnol 19:19–25. https://doi.org/10.1016/j.copbio.2007.10.009
Dunđerović D, Lipkovski JM, Boričic I et al (2015) Defining the value of CD56, CK19, Galectin 3 and HBME-1 in diagnosis of follicular cell derived lesions of thyroid with systematic review of literature. Diagn Pathol 10:196. https://doi.org/10.1186/s13000-015-0428-4
Bourboulia D, Jensen-Taubman S, Rittler MR et al (2011) Endogenous angiogenesis inhibitor blocks tumor growth via direct and indirect effects on tumor microenvironment. Am J Pathol 179:2589–2600. https://doi.org/10.1016/j.ajpath.2011.07.035
Wajner SM, Capp C, Brasil BA et al (2014) Reduced tissue inhibitor of metalloproteinase-2 expression is associated with advanced medullary thyroid carcinoma. Oncol Lett 7:731–737. https://doi.org/10.3892/ol.2013.1767
Tomita T (1997) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in thyroid C-cells and medullary thyroid carcinomas. Histopathology 31:150–156. https://doi.org/10.1046/j.1365-2559.1997.2160834.x
Chung H-K, Nam JS, Ahn CW et al (2016) Some elements in thyroid tissue are associated with more advanced stage of thyroid cancer in Korean women. Biol Trace Elem Res 171:54–62. https://doi.org/10.1007/s12011-015-0502-5
Şahin M, Karayakar F, Erdogan KE et al (2018) Liver tissue trace element levels in HepB patients and the relationship of these elements with histological injury in the liver and with clinical parameters. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS 45:70–77. https://doi.org/10.1016/j.jtemb.2017.09.014
Baltaci AK, Dundar TK, Aksoy F, Mogulkoc R (2017) Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biol Trace Elem Res 175:57–64. https://doi.org/10.1007/s12011-016-0768-2
Zabłocka-Słowińska K, Płaczkowska S, Prescha A et al (2018) Serum and whole blood Zn, Cu and Mn profiles and their relation to redox status in lung cancer patients. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS 45:78–84. https://doi.org/10.1016/j.jtemb.2017.09.024
Al-Sayer H, Mathew TC, Asfar S et al (2004) Serum changes in trace elements during thyroid cancers. Mol Cell Biochem 260:1–5. https://doi.org/10.1023/b:mcbi.0000026027.20680.c7
Przybylik-Mazurek E, Zagrodzki P, Kuźniarz-Rymarz S, Hubalewska-Dydejczyk A (2011) Thyroid disorders-assessments of trace elements, clinical, and laboratory parameters. Biol Trace Elem Res 141:65–75. https://doi.org/10.1007/s12011-010-8719-9
Martins LM, Barros IS, de Ferreira ES et al (1992) (2021) Expression of metalloproteinases 2 and 9 and plasma zinc concentrations in women with fibroadenoma. Rev Assoc Medica Bras 67:806–810. https://doi.org/10.1590/1806-9282.20201015
Belal A, Elanany MA, Santali EY et al (2022) Screening a panel of topical ophthalmic medications against MMP-2 and MMP-9 to investigate their potential in keratoconus management. Mol Basel Switz 27:3584. https://doi.org/10.3390/molecules27113584
Xu R, Chen M-Y, Liang W et al (2021) Zinc deficiency aggravation of ROS and inflammatory injury leading to renal fibrosis in mice. Biol Trace Elem Res 199:622–632. https://doi.org/10.1007/s12011-020-02184-x
Funding
Funding for open access charge: Universidad de Granada / CBUA. This study was supported by Zinc-Net “The Biology of Zinc”—COST action TD 1304 and the bilateral scientific cooperation between the Republic of Serbia and the Republic of Portugal -451–03-01765/2014–09/20. Héctor Vázquez-Lorente is under a FPU fellowship from the Spanish Ministry of Education with grant reference FPU18/03655.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by Héctor Vázquez-Lorente and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study abides by the Declaration of Helsinki on research involving human subjects and was approved by the Ethics committee of Faculty of Medicine, University of Belgrade No. 29/XI-10 for studies involving human subjects. Written informed consent was obtained from all individual participants after explaining goals of the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vázquez-Lorente, H., Dundjerović, D.M., Tatić, S.B. et al. Relationship between Trace Elements and Matrix Metalloproteinases 2 and 9 and their Tissue Inhibitors in Medullary Thyroid Carcinoma. Biol Trace Elem Res 201, 3225–3232 (2023). https://doi.org/10.1007/s12011-022-03431-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03431-z