Abstract
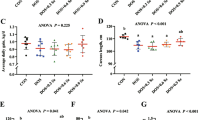
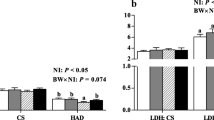
Intrauterine growth retardation (IUGR) causes oxidative stress in the skeletal muscle. Serine and selenoproteins are involved in anti-oxidative processes; however, whether IUGR affects selenium status and whether serine has beneficial effects remain elusive. Here, we investigated the effects of serine administration on selenium nutritional status and oxidative stress in the longissimus dorsi muscle of piglets with IUGR. Six newborn Min piglets having normal birth weight were administered saline, and 12 IUGR piglets were either administered saline or 0.8% serine. The results showed a lower selenium content in skeletal muscle in IUGR piglets, which was restored after serine administration. IUGR piglets showed a disturbed expression of genes encoding selenoproteins, with decreased expression of GPX2, GPX4, TXNRD1, and TXNRD3 and increased expression of DIO1, DIO2, SELF, SELM, SELP, and SELW. Notably, serine administration restored the expression levels of these genes. In accordance with the changes in gene expression, the activity of GPX, TXNRD, and DIO and the content of GSH and SELP were also altered, whereas serine administration restored their contents and activities. Moreover, we observed severe oxidative stress in the skeletal muscle of IUGR piglets, as indicated by decreased GSH content and increased MDA and PC content, whereas serine administration alleviated these changes. In conclusion, our results indicate that IUGR piglets showed a disturbed expression of genes encoding selenoproteins, accompanied by severe oxidative stress. Serine administration can improve selenium status, oxidative stress, and mitochondrial function in the longissimus dorsi muscle of piglets with IUGR. These results suggest that serine could potentially be used in the treatment of IUGR in piglets.


Similar content being viewed by others
Data Availability
Data will be made available on reasonable request.
References
McMillen IC, Robinson JS (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85(2):571–633. https://doi.org/10.1152/physrev.00053.2003
Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84(9):2316–2337. https://doi.org/10.2527/jas.2006-156
Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE (2010) Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci 88(13 Suppl):E195-204. https://doi.org/10.2527/jas.2009-2446
Widdowson EM (1971) Intra-uterine growth retardation in the pig. I. Organ size and cellular development at birth and after growth to maturity. Biol Neonate 19(4):329–40. https://doi.org/10.1159/000240427
Barker DJ (1998) In utero programming of chronic disease. Clin Sci (Lond) 95(2):115–128
Oksbjerg N, Nissen PM, Therkildsen M, Moller HS, Larsen LB, Andersen M, Young JF (2013) Meat Science and Muscle Biology Symposium: in utero nutrition related to fetal development, postnatal performance, and meat quality of pork. J Anim Sci 91(3):1443–1453. https://doi.org/10.2527/jas.2012-5849
Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17(9):571–588. https://doi.org/10.1016/j.jnutbio.2005.12.001
Cheng K, Wang T, Li S, Song Z, Zhang H, Zhang L, Wang T (2020) Effects of early resveratrol intervention on skeletal muscle mitochondrial function and redox status in neonatal piglets with or without intrauterine growth retardation. Oxid Med Cell Longev 2020:4858975. https://doi.org/10.1155/2020/4858975
Gueant JL, Elakoum R, Ziegler O, Coelho D, Feigerlova E, Daval JL, Gueant-Rodriguez RM (2014) Nutritional models of foetal programming and nutrigenomic and epigenomic dysregulations of fatty acid metabolism in the liver and heart. Pflugers Arch 466(5):833–850. https://doi.org/10.1007/s00424-013-1339-4
Hock MB, Kralli A (2009) Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71:177–203. https://doi.org/10.1146/annurev.physiol.010908.163119
Kieliszek M, Blazejak S (2016) Current knowledge on the importance of selenium in food for living organisms: a review. Molecules 21(5) https://doi.org/10.3390/molecules21050609
Duntas LH, Benvenga S (2015) Selenium: an element for life. Endocrine 48(3):756–775. https://doi.org/10.1007/s12020-014-0477-6
Chariot P, Bignani O (2003) Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve 27(6):662–668. https://doi.org/10.1002/mus.10304
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241. https://doi.org/10.1016/S0140-6736(00)02490-9
Kaur P, Bansal MP (2005) Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition 21(3):351–357. https://doi.org/10.1016/j.nut.2004.05.028
Zhang K, Zhao Q, Zhan T, Han Y, Tang C, Zhang J (2020) Effect of different selenium sources on growth performance, tissue selenium content, meat quality, and selenoprotein gene expression in finishing pigs. Biol Trace Elem Res 196(2):463–471. https://doi.org/10.1007/s12011-019-01949-3
Bizerea TO, Dezsi SG, Marginean O, Stroescu R, Rogobete A, Bizerea-Spiridon O, Ilie C (2018) The link between selenium, oxidative stress and pregnancy induced hypertensive disorders. Clin Lab 64(10):1593–1610. https://doi.org/10.7754/Clin.Lab.2018.180307
Wang Q, Sun LC, Liu YQ, Lu JX, Han F, Huang ZW (2016) The synergistic effect of serine with selenocompounds on the expression of SelP and GPx in HepG2 cells. Biol Trace Elem Res 173(2):291–296. https://doi.org/10.1007/s12011-016-0665-8
Amberg R, Mizutani T, Wu XQ, Gross HJ (1996) Selenocysteine synthesis in mammalia: an identity switch from tRNA(Ser) to tRNA(Sec). J Mol Biol 263(1):8–19. https://doi.org/10.1006/jmbi.1996.0552
Morrison DG, Dishart MK, Medina D (1988) Serine and methionine enhancement of selenite inhibition of DNA synthesis in a mouse mammary epithelial cell line. Carcinogenesis 9(10):1811–1815. https://doi.org/10.1093/carcin/9.10.1811
Gao X, Lee K, Reid MA, Sanderson SM, Qiu C, Li S, Liu J, Locasale JW (2018) Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep 22(13):3507–3520. https://doi.org/10.1016/j.celrep.2018.03.017
Long J, Liu Y, Zhou X, He L (2021) Dietary serine supplementation regulates selenoprotein transcription and selenoenzyme activity in pigs. Biol Trace Elem Res 199(1):148–153. https://doi.org/10.1007/s12011-020-02117-8
Zhou L, Feng Y, Liu Y, He L, Zhou X, Yin Y (2022) Serine supplementation in the diets of late gestating and lactating sows improves selenium nutritional status in sows and their offspring. Biol Trace Elem Res 200(2):609–614. https://doi.org/10.1007/s12011-021-02661-x
He L, Long J, Zhou X, Liu Y, Li T, Wu X (2020) Serine is required for the maintenance of redox balance and proliferation in the intestine under oxidative stress. FASEB J 34(3):4702–4717. https://doi.org/10.1096/fj.201902690R
Zhou X, He L, Wu C, Zhang Y, Wu X, Yin Y (2017) Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol Nutr Food Res 61(11) https://doi.org/10.1002/mnfr.201700262
Zhou X, Zhang Y, Wu X, Wan D, Yin Y (2018) Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early-weaned piglets. Cell Physiol Biochem 48(3):993–1002. https://doi.org/10.1159/000491967
Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ (2005) Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate 88(1):66–72. https://doi.org/10.1159/000084645
Zhang S, Xie Y, Li M, Yang H, Li S, Li J, Xu Q, Yang W, Jiang S (2020) Effects of different selenium sources on meat quality and shelf life of fattening pigs. Animals (Basel) 10(4) https://doi.org/10.3390/ani10040615
He L, Zhang H, Zhou X (2018) Weanling offspring of dams maintained on serine-deficient diet are vulnerable to oxidative stress. Oxid Med Cell Longev 2018:8026496. https://doi.org/10.1155/2018/8026496
Rashid CS, Bansal A, Simmons RA (2018) Oxidative stress, intrauterine growth restriction, and developmental programming of type 2 diabetes. Physiology (Bethesda) 33(5):348–359. https://doi.org/10.1152/physiol.00023.2018
Pendleton AL, Wesolowski SR, Regnault TRH, Lynch RM, Limesand SW (2021) Dimming the powerhouse: mitochondrial dysfunction in the liver and skeletal muscle of intrauterine growth restricted fetuses. Front Endocrinol (Lausanne) 12:612888. https://doi.org/10.3389/fendo.2021.612888
Cheng K, Ji S, Jia P, Zhang H, Wang T, Song Z, Zhang L, Wang T (2020) Resveratrol improves hepatic redox status and lipid balance of neonates with intrauterine growth retardation in a piglet model. Biomed Res Int 2020:7402645. https://doi.org/10.1155/2020/7402645
Ambrosio R, De Stefano MA, Di Girolamo D, Salvatore D (2017) Thyroid hormone signaling and deiodinase actions in muscle stem/progenitor cells. Mol Cell Endocrinol 459:79–83. https://doi.org/10.1016/j.mce.2017.06.014
Bianco AC (2013) Cracking the code for thyroid hormone signaling. Trans Am Clin Climatol Assoc 124:26–35
Ignacio DL, Silvestre DH, Anne-Palmer E, Bocco BM, Fonseca TL, Ribeiro MO, Gereben B, Bianco AC, Werneck-de-Castro JP (2017) Early developmental disruption of type 2 deiodinase pathway in mouse skeletal muscle does not impair muscle function. Thyroid 27(4):577–586. https://doi.org/10.1089/thy.2016.0392
Kilby MD, Verhaeg J, Gittoes N, Somerset DA, Clark PM, Franklyn JA (1998) Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR). J Clin Endocrinol Metab 83(8):2964–2971. https://doi.org/10.1210/jcem.83.8.5002
Drutel A, Archambeaud F, Caron P (2013) Selenium and the thyroid gland: more good news for clinicians. Clin Endocrinol (Oxf) 78(2):155–164. https://doi.org/10.1111/cen.12066
Hariharan S, Dharmaraj S (2020) Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology 28(3):667–695. https://doi.org/10.1007/s10787-020-00690-x
Kolukcu E, Atilgan D, Uluocak N, Deresoy FA, Katar M, Unsal V (2021) Milrinone ameliorates ischaemia-reperfusion injury in experimental testicular torsion/detorsion rat model. Andrologia 53(8):e14128. https://doi.org/10.1111/and.14128
Che L, Xuan Y, Hu L, Liu Y, Xu Q, Fang Z, Lin Y, Xu S, Wu D, Zhang K, Chen D (2015) Effect of postnatal nutrition restriction on the oxidative status of neonates with intrauterine growth restriction in a pig model. Neonatology 107(2):93–99. https://doi.org/10.1159/000368179
He J, Niu Y, Wang F, Wang C, Cui T, Bai K, Zhang J, Zhong X, Zhang L, Wang T (2018) Dietary curcumin supplementation attenuates inflammation, hepatic injury and oxidative damage in a rat model of intra-uterine growth retardation. Br J Nutr 120(5):537–548. https://doi.org/10.1017/S0007114518001630
Selak MA, Storey BT, Peterside I, Simmons RA (2003) Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab 285(1):E130–E137. https://doi.org/10.1152/ajpendo.00322.2002
Scarpulla RC (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813(7):1269–1278. https://doi.org/10.1016/j.bbamcr.2010.09.019
Liu J, Chen D, Yao Y, Yu B, Mao X, He J, Huang Z, Zheng P (2012) Intrauterine growth retardation increases the susceptibility of pigs to high-fat diet-induced mitochondrial dysfunction in skeletal muscle. PLoS ONE 7(4):e34835. https://doi.org/10.1371/journal.pone.0034835
Zhang H, Li Y, Su W, Ying Z, Zhou L, Zhang L, Wang T (2017) Resveratrol attenuates mitochondrial dysfunction in the liver of intrauterine growth retarded suckling piglets by improving mitochondrial biogenesis and redox status. Mol Nutr Food Res 61(5) https://doi.org/10.1002/mnfr.201600653
Funding
This work was financially supported by “Huxiang Young Talents Plan” Project of Hunan Province (2019RS2046), Youth Innovation Promotion Association CAS, and China Agriculture Research System of MOF and MARA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted according to the principles of the animal welfare committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences and was approved by the animal welfare committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, Y., Liu, Y., Guan, P. et al. Serine Administration Improves Selenium Status, Oxidative Stress, and Mitochondrial Function in Longissimus Dorsi Muscle of Piglets with Intrauterine Growth Retardation. Biol Trace Elem Res 201, 1740–1747 (2023). https://doi.org/10.1007/s12011-022-03304-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03304-5