Abstract
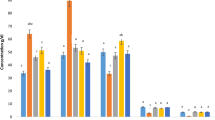
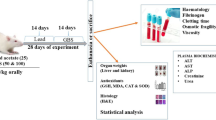
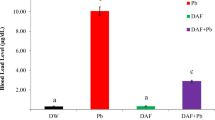
Lead (Pb) is a widespread environmental toxicant and its toxicity causes huge health impacts. The present study was conducted to examine the protective role of zinc (Zn) and calcium (Ca) supplements against bio-absorption of Pb in blood and organs including the liver and kidney. Hence, Sprague Dawley rats were divided in to five groups. G1 served as negative control and was provided with standard diet, G2 as positive control receiving standard diet + PbAc (20 mg/kg BW), G3 was provided with standard diet + PbAc (20 mg/kg BW) + ZnSO4 (20 mg/kg BW), G4 with standard diet + PbAc (20 mg/kg BW) + CaCO3 (7.5 g/kg BW) whereas G5 was fed on standard diet + PbAc (20 mg/kg BW) + ZnSO4 (20 mg/kg BW) + CaCO3 (7.5 g/kg BW). The salts were provided as solution, dissolved in 0.5 mL distilled water via orogastric tube. After 35 days, the overnight fasted rats were decapitated, and blood and organs were collected for analysis of levels of metals and liver and kidney function tests. The results depicted significant decrease in Pb concentration in blood and organs while increase in Zn and Ca absorption was observed as a result of Zn and Ca supplementation with Zn being better than Ca alone, specially however, combined effect of these supplements was more profound in improving liver and kidney stress biomarkers and maintained the normal architecture of renal and hepatic parenchyma. It was concluded that Zn and Ca co-supplementation hinder Pb absorption in blood, the liver, and kidney thus suggesting that their intake may protect from Pb toxicity.



Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Olakkaran S, Antony A, Purayil AK et al (2018) Lead modulated heme synthesis inducing oxidative stress mediated genotoxicity in Drosophila melanogaster. Sci Total Environ 634:628–639
Soliman HAM, Hamed M, Lee JS et al (2019) Protective effects of a novel pyrazolecarboxamide derivative against lead nitrate induced oxidative stress and DNA damage in Clarias gariepinus. Environ Pollut 247:678–684
Nariya A, Pathan A, Shah N et al (2018) Ameliorative effects of curcumin against lead induced toxicity in human peripheral blood lymphocytes culture. Drug Chem Toxicol 41:1–8
Shah F, Jain N (2016) Ameliorative action of synthetic and herbal antioxidants on lead induced hepatotoxicity: an in vitro study. Asian J Pharm Clin Res 9:364–370
Talpur S, Afridi HI, Kazi TG et al (2018) Interaction of lead with calcium, iron, and zinc in the biological samples of malnourished children. Biological Trace Elements Research 183:209–217
Shah F, Kazi TG, Afridi HI et al (2010) Environmental exposure of lead and iron deficit anemia in children age ranged 1–5 years: a cross sectional study. Sci Total Environ 408:5325–5330
Tariq U, Sadiq Butt M, Hameed A (2018) Probing the individual and synergistic effect of calcium and zinc against lead induced hematological alterations and oxidative stress. Annals of the Complementary and Alternative Medicine 1(1):1003
Ortega DR, Rodríguez PO, Pineda B et al (2020) Kynurenine pathway as a new target of cognitive impairment induced by lead toxicity during the lactation. Sci Rep 10:1–14
Huang S, Hu H, Sánchez BN et al (2016) Childhood blood lead levels and symptoms of attention deficit hyperactivity disorder (ADHD): a cross-sectional study of Mexican children. Environ Health Perspect 124:868–874
Gulson B, Mizon K, Taylor A et al (2019) Dietary zinc, calcium and nickel are associated with lower childhood blood lead levels. Environ Res 168:439–444
Aaseth J, Ajsuvakova OP, Skalny AV et al (2018) Chelator combination as therapeutic strategy in mercury and lead poisonings. Coord Chem Rev 358:1–12
Zhai Q, Yang L, Zhao J et al (2018) Protective effects of dietary supplements containing probiotics, micronutrients, and plant extracts against lead toxicity in mice. Front Microbiol 9:2134
Chaudhari M, Hussain S, Rehman H et al (2019) A perspective study on lead poisoning: exposure, effects and treatment. Int. J Econ Environ Geol 10:70–77
Kordas K, Burganowski R, Roy A et al (2018) Nutritional status and diet as predictors of children’s lead concentrations in blood and urine. Environ Int 111:43–51
Jarosz M, Olbert M, Wyszogrodzka G et al (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling Inflammopharmacology 25:11–24
Rădulescu A, Lundgren S (2019) A pharmacokinetic model of lead absorption and calcium competitive dynamics. Sci Rep 9:1–27
Arlington V (1990) Association of official analytical chemists: Washington. WA, USA
Wani AL, Ahmad A, Shadab G et al (2017) Possible role of zinc in diminishing lead-related occupational stress—a zinc nutrition concern. Environ Sci Pollut Res 24:8682–8691
Martín-Lagos F, Navarro-Alarcón M, Terrés-Martos C et al (1998) Serum zinc levels in healthy subjects from southeastern Spain. Biol Trace Elem Res 61:51–60
Shahsavani D, Baghshani H, Alishahi E (2011) Efficacy of allicin in decreasing lead (Pb) accumulation in selected tissues of lead-exposed common carp (Cyprinus carpio). Biol Trace Elem Res 142:572–580
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Klein B, Kaufman JH (1967) Automated alkaline phosphatase determination: III. Evaluation of phenolphthalein monophosphate Clinical Chemistry 13:290–298
Fawcett J, Scott J (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
de Faria LC, and Pasquini C (1992) Spectrophotometric determination of creatinine by monosegmented continuous-flow analysis. J Autom Chem 14
Tariq, U., Butt, M.S., Pasha, I. et al. (2021) Neuroprotective effects of Olea europaea L. fruit extract against cigarette smoke‐induced depressive‐like behaviors in Sprague–Dawley rats. Journal of Food Biochemistry 45:e14014.
Zhang Y, Li Q, Liu X et al (2013) Antioxidant and micronutrient-rich milk formula reduces lead poisoning and related oxidative damage in lead-exposed mice. Food Chem Toxicol 57:201–208
Briner W (2014) The Alchemist’s approach to metal poisoning: transforming the metal burden. Toxics 2:364–376
Ettinger AS, Lamadrid-Figueroa H, Téllez-Rojo MM et al (2009) Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo-controlled trial. Environ Health Perspect 117:26–31
Cobbina SJ, Chen Y, Zhou Z et al (2015) Low concentration toxic metal mixture interactions: effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure. Chemosphere 132:79–86
Jiao J, Lü G, Liu X et al (2011) Reduction of blood lead levels in lead-exposed mice by dietary supplements and natural antioxidants. J Sci Food Agric 91:485–491
Hanan AA, Riham MR (2012) Effect of lead toxicity on cytogenisity, biochemical constituents and tissue residue with protective role of activated charcoal and casein in male rats. Aust J Basic Appl Sci 6:497–509
Sarić MM, Blanuša M, Piasek M et al (2002) Effect of dietary calcium on cadmium absorption and retention in suckling rats. Biometals 15:175–182
Dai W, Fu L, Du H et al (2009) Changes in growth performance, metabolic enzyme activities, and content of Fe, Cu, and Zn in liver and kidney of tilapia (Oreochromis niloticus) exposed to dietary Pb. Biol Trace Elem Res 128:176–183
Kasperczyk A, Prokopowicz A, Dobrakowski M et al (2012) The effect of occupational lead exposure on blood levels of zinc, iron, copper, selenium and related proteins. Biological Tace Element Research 150:49–55
Roohani N, Hurrell R, Kelishadi R et al (2013) Zinc and its importance for human health: an integrative review. J Res Med Sci 18:144
Garza, A., Vega, R. and Soto, E. (2006) Cellular mechanisms of lead neurotoxicity. Medical Science Monitor, 12:RA57-RA65.
Zhang Z, Gao X, Guo M et al (2017) The protective effect of baicalin against lead-induced renal oxidative damage in mice. Biol Trace Elem Res 175:129–135
Obeng-Gyasi E, Armijos RX, Weigel MM et al (2018) Hepatobiliary-related outcomes in US adults exposed to lead. Environments 5:46
Mazumdar I, Goswami K (2014) Chronic exposure to lead: a cause of oxidative stress and altered liver function in plastic industry workers in Kolkata, India. Indian J Clin Biochem 29:89–92
Conceição E, Moura E, Soares P et al (2016) High calcium diet improves the liver oxidative stress and microsteatosis in adult obese rats that were overfed during lactation. Food Chem Toxicol 92:245–255
Katayama K, Kawaguchi T, Shiraishi K et al (2018) The prevalence and implication of zinc deficiency in patients with chronic liver disease. Journal of Clinical Medicine Research 10:437
Oyagbemi AA, Omobowale TO, Akinrinde AS et al (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30:1235–1243
Alwaleedi SA (2016) Hematobiochemical changes induced by lead intoxication in male and female albino mice. Int J Physiol Pharm Pharmacol 6:46–51
Salmean YA, Segal MS, Langkamp-Henken B et al (2013) Foods with added fiber lower serum creatinine levels in patients with chronic kidney disease. J Ren Nutr 23:29–32
Amah UK, Madu NK, Ahaneku JE et al (2017) Evaluation of nephrotoxic effect of lead exposure among automobile repairers in Nnewi Metropolis. Int J Res Med Sci 2:1107–1111
Hou G, Surhio MM, Ye H et al (2019) Protective effects of a Lachnum polysaccharide against liver and kidney injury induced by lead exposure in mice. Int J Biol Macromol 124:716–723
Sujatha K, Srilatha C, Anjaneyulu Y et al (2011) Lead acetate induced nephrotoxicity in Wistar albino rats, pathological, immunohistochemical and ultra structural studies. Int J Pharm Biol Sci 2:459-B469
Amin, I., Hussain, I., Rehman, M.U. et al. (2021) Zingerone prevents lead‐induced toxicity in liver and kidney tissues by regulating the oxidative damage in Wistar rats. Journal of Food Biochemistry 45:e13241.
Kucukler S, Benzer F, Yildirim S et al (2021) Protective effects of chrysin against oxidative stress and inflammation induced by lead acetate in rat kidneys: a biochemical and histopathological approach. Biol Trace Elem Res 199:1501–1514
Soussi A, Gargouri M, El Feki A (2018) Effects of co-exposure to lead and zinc on redox status, kidney variables, and histopathology in adult albino rats. Toxicol Ind Health 34:469–480
Al-Qahtani WS, Virk P (2015) Effect of calcium supplementation on lead-induced genotoxicity and bioaccumulation in suckling Wistar rats. Toxicol Environ Chem 97:968–988
Author information
Authors and Affiliations
Contributions
Masood Sadiq Butt provided technical assistance in designing the study. Urwa Tariq conducted the research and gathered the data for manuscript. Iahtisham-Ul-Haq applied statistical analysis, interpreted the results, and prepared the manuscript. Komal Javed reviewed the manuscript and helped in the preparation of final draft. All the authors contributed sufficiently in making the manuscript technically sound and then approved the manuscript for publication.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study was approved by the Directorate of Advance Studies, University of Agriculture, Faisalabad, Pakistan, after compliance with the research/ethical standards from the scrutiny committee of the NIFSAT, UAF.
Consent for Publication
All the authors have consented for publication of this research.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Butt, M.S., Iahtisham-Ul-Haq, Javed, K. et al. Co-supplementation of Zinc and Calcium Suppresses Bio-absorption of Lead in Sprague Dawley Rats. Biol Trace Elem Res 201, 1317–1326 (2023). https://doi.org/10.1007/s12011-022-03233-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03233-3