Abstract
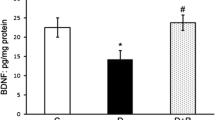
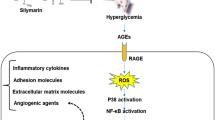
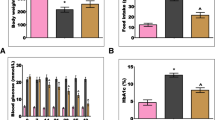
Diabetes mellitus induces optic nerve injury via the excessive generation of mitochondria reactive free oxygen radical (mitROS). TRPM2 channel is activated by mitROS, although it is inhibited by selenium (Se) and resveratrol (RSV). The activation of TRPM2 induces apoptosis and oxidative injury in the optic nerve. The inhibition of TRPM2 may decrease the optic nerve injury action of diabetes mellitus after the treatments of Se and RSV. Present study aimed to investigate the protective actions of Se and RSV on the excessive Ca2+ influx and mitROS generation-mediated optic nerve oxidative injury via the modulation of TRPM2. Fifty-six C57BL/6j male mice were divided into seven groups as control, Se, RSV, streptozotocin (STZ), STZ + Se, STZ + RSV, and STZ + Se + RSV. The STZ-mediated stimulation of TRPM2 increased the cytosolic Ca2+, lipid peroxidation, mitROS, cytosolic ROS, apoptosis, caspase-3, caspase-8, and caspase-9 concentrations in the mice, although their concentrations were decreased in the optic nerve by the treatments of Se and RSV. The STZ-induced decrease of optic nerve viability, glutathione, glutathione peroxidase, vitamin A, and vitamin E concentrations was also upregulated by the treatments of Se and RSV. The STZ-induced increase of TRPM2, PARP-1, caspase-3, and caspase-9 protein band expressions was diminished by the treatments of Se and RSV. In conclusion, STZ induced the optic nerve oxidative injury and apoptosis via the upregulation of TRPM2 stimulation, although the treatments of Se and RSV decreased the injury and apoptosis via the downregulation of TRPM2 activity.
Graphical abstract









Similar content being viewed by others
Data Availability
Row data and analyzed results are available from the corresponding author.
Abbreviations
- 2/APB:
-
2-Aminoethoxydiphenyl borate
- ACA:
-
N-(p-Amylcinnamoyl)anthranilic acid
- ADPR:
-
ADP-ribose
- BF:
-
Bright field
- Ca2 + :
-
Calcium ion
- CASP/3:
-
Caspase-3
- CASP/8:
-
Caspase-8
- CASP/9:
-
Caspase-9
- CLSM:
-
Confocal laser scanning microscope
- cytCa2 + :
-
Cytosolic free calcium ion
- cytROS:
-
Cytosolic free reactive oxygen radicals
- DM:
-
Diabetes mellitus
- DRG:
-
Dorsal root ganglion
- GSH:
-
Glutathione
- GSH/Px:
-
Glutathione peroxidase
- MDA:
-
Lipid peroxidation
- mitPOT:
-
Mitochondrial membrane potential
- mitROS:
-
Mitochondrial free reactive oxygen radicals
- PARP1:
-
Poly(ADP-ribose)polymerase-1
- RSV:
-
Resveratrol
- Se:
-
Selenium
- TRP:
-
Transient receptor potential
- TRPM2:
-
Transient receptor potential melastatin 2
References
Jeganathan VS, Wang JJ, Wong TY (2008) Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care 31(9):1905–1912. https://doi.org/10.2337/dc08-0342
Chang KC, Liu PF, Chang CH, Lin YC, Chen YJ, Shu CW (2022) The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases. Cell Biosci 12(1):1. https://doi.org/10.1186/s13578-021-00736-9
Mendonca HR, Carpi-Santos R, da Costa CK, Blanco Martinez AM (2020) Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res 15(4):625–635. https://doi.org/10.4103/1673-5374.266910
Kowluru RA, Mishra M (2015) Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta 1852(11):2474–2483. https://doi.org/10.1016/j.bbadis.2015.08.001
Özkaya D, Nazıroğlu M (2020) Curcumin diminishes cisplatin-induced apoptosis and mitochondrial oxidative stress through inhibition of TRPM2 channel signaling pathway in mouse optic nerve. J Recept Signal Transduct Res 40(2):97–108. https://doi.org/10.1080/10799893.2020.1720240
Ha Y, Saul A, Tawfik A, Zorrilla EP, Ganapathy V, Smith SB (2012) Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol Vis 18:2860–2870
Saadane A, Du Y, Thoreson WB, Miyagi M, Lessieur EM, Kiser J, Wen X, Berkowitz BA, Kern TS (2021) Photoreceptor cell calcium dysregulation and calpain activation promote pathogenic photoreceptor oxidative stress and inflammation in prodromal diabetic retinopathy. Am J Pathol 191(10):1805–1821. https://doi.org/10.1016/j.ajpath.2021.06.006
Chang JY, Yu F, Shi L, Ko ML, Ko GY (2019) Melatonin affects mitochondrial fission/fusion dynamics in the diabetic retina. J Diabetes Res 2019:8463125. https://doi.org/10.1155/2019/8463125
Özkaya D, Nazıroğlu M, Vanyorek L, Muhamad S (2021) Involvement of TRPM2 channel on hypoxia-induced oxidative injury, inflammation, and cell death in retinal pigment epithelial cells: modulator action of selenium nanoparticles. Biol Trace Elem Res 199(4):1356–1369. https://doi.org/10.1007/s12011-020-02556-3
Li X, Zhao H, Wang Q, Liang H, Jiang X (2015) Fucoidan protects ARPE-19 cells from oxidative stress via normalization of reactive oxygen species generation through the Ca2+-dependent ERK signaling pathway. Mol Med Rep 11(5):3746–3752. https://doi.org/10.3892/mmr.2015.3224
Choi HJ, Sun D, Jakobs TC (2015) Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol Vis 21:749–766
Nazıroğlu M, Lückhoff A (2012) A calcium influx pathway regulated separately by oxidative stress and ADP-Ribose in TRPM2 channels: single channel events. Neurochem Res 33(7):1256–1262. https://doi.org/10.1007/s11064-007-9577-5
Perraud AL, Fleig A, Dunn CA et al (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411(6837):595–9. https://doi.org/10.1038/35079100
Hara Y, Wakamori M, Ishii M et al (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9(1):163–173. https://doi.org/10.1016/S1097-2765(01)00438-5
Kahya MC, Nazıroğlu M, Övey İS (2017) Modulation of diabetes-induced oxidative stress, apoptosis, and Ca2+ entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol 54(3):2345–2360. https://doi.org/10.1007/s12035-016-9727-3
Abuarab N, Munsey TS, Jiang LH, Li J, Sivaprasadarao A (2017) High glucose-induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn2+-mediated mitochondrial fission. Sci Signal 10(490):eaal4161. https://doi.org/10.1126/scisignal.aal4161
Tseng HH, Vong CT, Kwan YW, Lee SM, Hoi MP (2016) TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells. Sci Rep 6:35016. https://doi.org/10.1038/srep35016
McNulty S, Fonfria E (2005) The role of TRPM channels in cell death. Pflugers Arch 451(1):235–242. https://doi.org/10.1007/s00424-005-1440-4
González de Vega R, García M, Fernández-Sánchez ML, González-Iglesias H, Sanz-Medel A (2018) Protective effect of selenium supplementation following oxidative stress mediated by glucose on retinal pigment epithelium. Metallomics 10(1):83–92. https://doi.org/10.1039/c7mt00209b
Kowluru RA, Koppolu P (2002) Diabetes-induced activation of caspase-3 in retina: effect of antioxidant therapy. Free Radic Res 36(9):993–999. https://doi.org/10.1080/1071576021000006572
Di Leo MA, Ghirlanda G, Gentiloni Silveri N, Giardina B, Franconi F, Santini SA (2003) Potential therapeutic effect of antioxidants in experimental diabetic retina: a comparison between chronic taurine and vitamin E plus selenium supplementations. Free Radic Res 37(3):323–330. https://doi.org/10.1080/1071576021000055271
Yıldızhan K, Nazıroğlu M (2021) Protective role of selenium on MPP+ and homocysteine-induced TRPM2 channel activation in SH-SY5Y cells. J Recept Signal Transduct Res 1–10. https://doi.org/10.1080/10799893.2021.1981381
Akyuva Y, Nazıroğlu M, Yıldızhan K (2021) Selenium prevents interferon-gamma induced activation of TRPM2 channel and inhibits inflammation, mitochondrial oxidative stress, and apoptosis in microglia. Metab Brain Dis 36(2):285–298. https://doi.org/10.1007/s11011-020-00624-0
Toro MD, Nowomiejska K, Avitabile T, Rejdak R, Tripodi S, Porta A, Reibaldi M, Figus M, Posarelli C, Fiedorowicz M (2019) Effect of resveratrol on in vitro and in vivo models of diabetic retinophathy: a systematic review. Int J Mol Sci 20(14):3503. https://doi.org/10.3390/ijms20143503
Öztürk Y, Günaydın C, Yalçın F, Nazıroğlu M, Braidy N (2019) Resveratrol enhances apoptotic and oxidant effects of paclitaxel through TRPM2 channel activation in DBTRG glioblastoma cells. Oxid Med Cell Longev 2019:4619865. https://doi.org/10.1155/2019/4619865
Akyuva Y, Nazıroğlu M (2020) Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci Rep 10(1):6449. https://doi.org/10.1038/s41598-020-63577-5
Yildizhan K, Çinar R, Naziroğlu M (2022) The involvement of TRPM2 on the MPP+-induced oxidative neurotoxicity and apoptosis in hippocampal neurons from neonatal mice: protective role of resveratrol. Neurol Res 1–9. https://doi.org/10.1080/01616412.2022.2027644
Al-Hussaini H, Kittaneh RS, Kilarkaje N (2021) Effects of trans-resveratrol on type 1 diabetes-induced up-regulation of apoptosis and mitogen-activated protein kinase signaling in retinal pigment epithelium of Dark Agouti rats. Eur J Pharmacol 904:174167. https://doi.org/10.1016/j.ejphar.2021.174167
Cong P, Wang T, Tong C, Liu Y, Shi L, Mao S, Shi X, Jin H, Liu Y, Hou M (2021) Resveratrol ameliorates thoracic blast exposure-induced inflammation, endoplasmic reticulum stress and apoptosis in the brain through the Nrf2/Keap1 and NF-κB signaling pathway. Injury 52(10):2795–2802. https://doi.org/10.1016/j.injury.2021.08.019
Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL (2015) NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest 125(4):1433–1445
Quill B, Irnaten M, Docherty NG, McElnea EM, Wallace DM, Clark AF, O’Brien CJ (2015) Calcium channel blockade reduces mechanical strain-induced extracellular matrix gene response in lamina cribrosa cells. Br J Ophthalmol 99(7):1009–1014. https://doi.org/10.1136/bjophthalmol-2014-306093
Ertilav K (2019) Levetiracetam modulates hypoxia-induced inflammation and oxidative stress via inhibition of TRPV1 channel in the DBTRG glioblastoma cell line. J Cell Neurosci Oxid Stress 11(3):885–894. https://doi.org/10.37212/jcnos.715227
Akyuva Y (2020) Clostridium botulinum neurotoxin A inhibits DBTRG glioblastoma cell proliferation and TRPV1 channel signaling pathways. J Cell Neurosci Oxid Stress 12(1):903–913. https://doi.org/10.37212/jcnos.809635
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Analytical Biochem 16:359–364
Sedlak J, Lindsay RHC (1968) Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochem 25:192–205
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Com 71:952–958
Desai ID (1980) Vitamin E analysis methods for animal tissues. Methods Enzymol 105:138–147
Suzuki J, Katoh N (1990) A simple and cheap method for measuring vitamin A in cattle using only a spectrophotometer. Jpn J Vet Sci 52:1282–1284
Nazıroğlu M, Dilsiz N, Cay M (1999) Protective role of intraperitoneally administered vitamins C and E and selenium on the levels of lipid peroxidation in the lens of rats made diabetic with streptozotocin. Biol Trace Elem Res 70(3):223–232. https://doi.org/10.1007/BF02783831
Pan YR, Song JY, Fan B, Wang Y, Che L, Zhang SM, Chang YX, He C, Li GY (2020) mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Commun Signal 18(1):27. https://doi.org/10.1186/s12964-019-0498-0
Nazıroğlu M (2022) A novel antagonist of TRPM2 and TRPV4 channels: carvacrol. Metab Brain Dis 1–18. https://doi.org/10.1007/s11011-021-00887-1
Blenn C, Wyrsch P, Bader J, Bollhalder M, Althaus FR (2011) Poly(ADP-ribose)glycohydrolase is an upstream regulator of Ca2+ fluxes in oxidative cell death. Cell Mol Life Sci 68(8):1455–1466. https://doi.org/10.1007/s00018-010-0533-1
Sun Y, Sukumaran P, Selvaraj S, Cilz NI, Schaar A, Lei S, Singh BB (2018) (2018) TRPM2 Promotes Neurotoxin MPP+/MPTP-Induced Cell Death. Mol Neurobiol 55(1):409–420. https://doi.org/10.1007/s12035-016-0338-9
Uchida K, Dezaki K, Damdindorj B, Inada H, Shiuchi T, Mori Y, Yada T, Minokoshi Y, Tominaga M (2011) Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes 60(1):119–126. https://doi.org/10.2337/db10-0276
Carrasco C, Rodríguez BA, Pariente JA (2015) Melatonin as a stabilizer of mitochondrial function: Role in diseases and aging. Turk J Biol 39:822–831
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658. https://doi.org/10.1111/j.1471-4159.2006.03907.x.47
Ekicier Acar S, Sarıcaoğlu MS, Çolak A, Aktaş Z, SepiciDinçel A (2020) Neuroprotective effects of topical coenzyme Q10 + vitamin E in mechanic optic nerve injury model. Eur J Ophthalmol 30(4):714–722. https://doi.org/10.1177/1120672119833271
Liu WY, Liou SS, Hong TY, Liu IM (2017) Protective effects of hesperidin (citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients 9(12):1312. https://doi.org/10.3390/nu9121312
Khorsand M, Akmali M, Akhzari M (2019) Efficacy of melatonin in restoring the antioxidant status in the lens of diabetic rats induced by streptozotocin. J Diabetes Metab Disord 18(2):543–549. https://doi.org/10.1007/s40200-019-00445-8
Li Q, Hueckstaedt LK, Ren J (2009) The protease inhibitor UCF-101 ameliorates streptozotocin-induced mouse cardiomyocyte contractile dysfunction in vitro: role of AMP-activated protein kinase. Exp Physiol 94(9):984–994. https://doi.org/10.1113/expphysiol.2009.049189
Funding
The present study was financially supported by BSN Health, Analyses, Innov., Consult., Org., Agricul., Industry LTD., Isparta, Turkey. (The number of the project is: 2021–05. The owner of the project is Dr. Hatice Daldal.)
Author information
Authors and Affiliations
Contributions
Hatice Daldal and Mustafa Nazıroğlu planned the present hypothesis and the main manuscript text. The optic nerve isolation, cell culture, antioxidant, and cell viability analyses in the current study were performed by Hatice Daldal. The remaining analyses and graphic presentations were performed by Mustafa Nazıroğlu.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest. There is no human sample in the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daldal, H., Nazıroğlu, M. Selenium and Resveratrol Attenuated Diabetes Mellitus-Mediated Oxidative Retinopathy and Apoptosis via the Modulation of TRPM2 Activity in Mice. Biol Trace Elem Res 200, 2283–2297 (2022). https://doi.org/10.1007/s12011-022-03203-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03203-9