Abstract
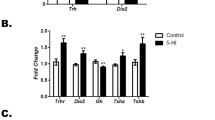
Excess iodine can cause autoimmune thyroiditis (AIT) in women, but it is unclear whether this has any implications for neurodevelopmental mechanisms in offspring. We studied the effects of experimental autoimmune thyroiditis (EAT) rats with different amounts of iodine intake on offspring brain development via the brain-derived neurotrophic factor (BDNF)-tropomycin receptor kinase B (TrkB) signaling pathway, because BDNF plays an important role in neurodevelopment. Rats in three thyroglobulin (Tg) immunized groups with varying iodine intakes (Tg (100 µg/L iodine), Tg + High-iodine I group (Tg + HI, 20 mg/L iodine), and Tg + High-iodine II group (Tg + HII, 200 mg/L iodine)) were injected with 800 µg Tg once every 2 weeks for 3 times. Rats in the control group (NI, 100 µg/L iodine) were immunized with saline. Arsenic-cerium catalytic spectrophotometry was used to measure urine iodine levels. The lymphocytic infiltration in the thyroids was observed by histopathological studies. Thyroid autoantibodies levels were measured using radioimmunoassay. The norepinephrine (NE) contents were measured by an enzyme-linked immunosorbent assay. The levels of the BDNF-TrkB signaling pathway and related genes were measured by quantitative real-time PCR and Western blot. Urinary iodine levels increased as iodine intake increased. Lymphocytes were significantly aggravated in Tg-immunized rats. Serum thyroglobulin antibody (TgAb) and thyroid peroxidase antibody (TPOAb) levels were clearly elevated in Tg-immunized rats. Tg-immune groups had significantly lower NE levels. The BDNF-TrkB signaling pathway and related gene mRNA and protein levels were found to be significantly lower in Tg-immune groups with higher iodine levels. Maternal AIT may reduce the levels of certain neurodevelopmental mechanisms in the offspring, such as the BDNF-TrkB signaling pathway and related factors, while excessive iodine consumption by the mother may exacerbate this effect.




Similar content being viewed by others
Data Availability
Raw data from animals involved in this study will be kept confidential and will not be shared.
References
Redman K, Ruffman T, Fitzgerald P, Skeaffp S (2016) Iodine deficiency and the brain: effects and mechanisms. Crit Rev Food Sci Nutr 56(16):2695–2713
Guo Q, Wu D, Fan C, Peng S, Guan H, Shan Z, Teng W (2018) Iodine excess did not affect the global DNA methylation status and DNA methyltransferase expression in T and B lymphocytes from NOD.H-2(h4) and Kunming mice. Int Immunopharmacol 55:151–157
Leung AM, Braverman LE (2014) Consequences of excess iodine. Nat Rev Endocrinol 10(3):136–142
Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, Bai X, Li Y, Li N, Li Z, Wang S, Xing Q, Xue H, Zhu L, Hou X, Fan C, Teng W (2011) More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 164(6):943–950
Teng X, Shan Z, Teng W, Fan C, Wang H, Guo R (2009) Experimental study on the effects of chronic iodine excess on thyroid function, structure, and autoimmunity in autoimmune-prone NOD.H-2h4 mice. Clin Exp Med 9(1):51–9
Dong YH, Fu DG (2014) Autoimmune thyroid disease: mechanism, genetics and current knowledge. Eur Rev Med Pharmacol Sci 18(23):3611–3618
Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, Churilov LP, Ferrari SM, Antonelli A (2019) Hashimotos’ thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab 33(6):101367
Freire C, Ramos R, Amaya E, Fernández MF, Santiago-Fernández P, Lopez-Espinosa MJ, Arrebola JP, Olea N (2010) Newborn TSH concentration and its association with cognitive development in healthy boys. Eur J Endocrinol 163(6):901–909
Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, Teng X, Guo R, Wang H, Li J, Chen Y, Wang W, Chawinga M, Zhang L, Yang L, Zhao Y, Hua T (2010) Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol 72(6):825–829
Pérez-Lobato R, Ramos R, Arrebola JP, Calvente I, Ocón-Hernández O, Dávila-Arias C, Pérez-García M, Olea N, Fernández MF (2015) Thyroid status and its association with cognitive functioning in healthy boys at 10 years of age. Eur J Endocrinol 172(2):129–139
Gao TS, Teng WP, Shan ZY, Jin Y, Guan HX, Teng XC, Yang F, Wang WB, Shi XG, Tong YJ, Li D, Chen W (2004) Effect of different iodine intake on schoolchildren’s thyroid diseases and intelligence in rural areas. Chin Med J 117(10):1518–1522
Liu HL, Lam LT, Zeng Q, Han SQ, Fu G, Hou CC (2009) Effects of drinking water with high iodine concentration on the intelligence of children in Tianjin, China. J Public Health (Oxf) 31(1):32–38
Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF (2009) Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect 117(12):1953–1958
Trumpff C, De Schepper J, Vanderfaeillie J, Vercruysse N, Van Oyen H, Moreno-Reyes R, Tafforeau J, Vandevijvere S (2016) Neonatal thyroid-stimulating hormone concentration and psychomotor development at preschool agep. Arch Dis Child 101(12):1100–1106
Li F, Wan S, Zhang L, Li B, He Y, Shen H, Liu L (2021) A meta-analysis of the effect of iodine excess on the intellectual development of children in areas with high iodine levels in their drinking water. Biol Trace Elem Res 200(4):1580–1590
Cowansage KK, LeDoux JE, Monfils MH (2010) Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol 3(1):12–29
Patapoutian A, Reichardt LF (2001) Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11(3):272–280
Amidfar M, de Oliveira J, Kucharska E, Budni J, Kim YK (2020) The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci 257:118020
Song JH, Yu JT, Tan L (2015) Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol Neurobiol 52(3):1477–1493
Churilov LP, Sobolevskaia PA, Stroev YI (2019) Thyroid gland and brain: enigma of Hashimoto’s encephalopathy. Best Pract Res Clin Endocrinol Metab 33(6):101364
Liontiris MI, Mazokopakis EE (2017) A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients Points that need more investigation. Hell J Nucl Med 20(1):51–56
Cui SL, Yu J, Shoujun L (2014) Iodine Intake increases IP-10 expression in the serum and thyroids of rats with experimental autoimmune thyroiditis. Int J Endocrinol 2014:581069
Li J (2018) Neuroprotective effect of (-)-epigallocatechin-3-gallate on autoimmune thyroiditis in a rat model by an anti-inflammation effect, anti-apoptosis and inhibition of TRAIL signaling pathway. Exp Ther Med 15(1):1087–1092
Yang W, Xiang Y, Zhang H, Shan Z, Li J, Teng W (2020) The role of protein disulphide-isomerase A3 as autoantigen in the pathogenesis of autoimmune thyroiditis and related brain damage in adult mice. Clin Immunol (Orlando, Fla.) 212:108350
Ghassabian A, Henrichs J, Tiemeier H (2014) Impact of mild thyroid hormone deficiency in pregnancy on cognitive function in children: lessons from the Generation R Stud. Best Pract Res Clin Endocrinol Metab 28(2):221–32
Song M, Martinowich K, Lee FS (2017) BDNF at the synapse: why location matters. Mol Psychiatry 22(10):1370–1375
Sasi M, Vignoli B, Canossa M, Blum R (2017) Neurobiology of local and intercellular BDNF signaling. Pflugers Arch 469(5–6):593–610
Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G (2020) BDNF as a promising therapeutic agent in Parkinson’s disease. Int J Mol Sci 21(3):1170
Colucci-D’Amato L, Speranza L, Volpicelli F (2020) Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci 21(20):7777
Kamyshna LB II, Pavlovych LP, Sydorchuk IV, Malyk AMK (2021) BDNF blood serum linkage with BDNF gene polymorphism (rs6265) in thyroid pathology patients in the West-Ukrainian population. Endocr Regul 55(4):193–203
Massolt ET, Effraimidis G, Korevaar TI, Wiersinga WM, Visser WE, Peeters RP, Drexhage HA (2016) Aberrant levels of hematopoietic/neuronal growth and differentiation factors in euthyroid women at risk for autoimmune thyroid disease. PloS one 11(4):e0153892
Saboory E, Ghasemi M, Mehranfard N (2020) Norepinephrine, neurodevelopment and behavior. Neurochem Int 135:104706
Wang X, Liu H, Zhang Y, Li J, Teng X, Liu A, Yu X, Shan Z, Teng W (2015) Effects of isolated positive maternal thyroglobulin antibodies on brain development of offspring in an experimental autoimmune thyroiditis model. Thyroid: Off J Am Thyroid Assoc 25(5):551–558
Zhang L, Teng W, Liu Y, Li J, Mao J, Fan C, Wang H, Zhang H, Shan Z (2012) Effect of maternal excessive iodine intake on neurodevelopment and cognitive function in rat offspring. BMC Neurosci 13:121
Slobodin B, Han R, Calderone V, Vrielink J, Loayza-Puch F, Elkon R, Agami R (2017) Transcription impacts the efficiency of mRNA translation via co-transcriptional N6-adenosine methylation. Cell 169(2):326-337.e12
Miranda A, Sousa N (2018) Maternal hormonal milieu influence on fetal brain development. Brain Behav 8(2):e00920
Kodomari I, Wada E, Nakamura S, Wada K (2009) Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem Int 54(2):95–98
Rayman MP (2019) Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc 78(1):34–44
Montagna G, Imperiali M, Agazzi P, D’Aurizio F, Tozzoli R, Feldt-Rasmussen U, Giovanella L (2016) Hashimoto’s encephalopathy: a rare proteiform disorder. Autoimmun Rev 15(5):466–476
Acknowledgements
We thank Prof. Hongmei Shen and Ms. Lixiang Liu and other researchers for their help, and the Key Laboratory of Pathogenesis and Epidemiology, Harbin Medical University (23618504) for providing experimental equipment and environment.
Funding
The study was supported by the National Natural Science Foundation of China (Grant No. 81703175).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Hongmei Shen, Lixiang Liu; Performed the experiments: Meihui Jin, Zheng Zhou, Li Zhang, Yao Chen; Established the mouse model: Meihui Jin, Zheng Zhou; Analyzed the data: Meihui Jin; Drafted the manuscript: Meihui Jin, Hongmei Shen, Lixiang Liu.
All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The study was approved by the Medical Ethics Committee of the Endemic Disease Control Centre of Harbin Medical University (hrbmuecdc20170305) and complied with animal ethical requirements.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jin, M., Zhou, Z., Zhang, L. et al. Effects of Excessive Iodine on the BDNF-TrkB Signaling Pathway and Related Genes in Offspring of EAT Rats. Biol Trace Elem Res 201, 776–785 (2023). https://doi.org/10.1007/s12011-022-03187-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03187-6