Abstract
The present study aimed to investigate the effects of feeding zinc (Zn)-biofortified wheat on performance, digestibility, and concentrations of minerals in quails. Zinc biofortification of wheat has been realized in the field by ergonomically applying Zn to foliar two and three times, which increased grain Zn from 18 mg/kg (control) to 34 and 64 mg/kg. A total of 180 quails were divided into six groups, each containing 30 birds, and fed diets containing wheat grain with either 18, 34, or 64 mg/kg with or without zinc picolinate (ZnPic) supplementation. Bodyweight, feed intake, feed efficiency, and cold carcass weights were greater (P = 0.0001) when the quails were fed a diet containing the biofortified wheat-containing 64 mg Zn/kg. Nitrogen, ash, Ca, P, Zn, Cu, and Fe retentions were greater with the Zn-biofortified wheat-containing 64 mg Zn/kg (P ≤ 0.026). The nutrient excretions were low with feeding a diet containing biofortified wheat-containing 64 mg Zn/kg (P ≤ 0.023). Serum, liver, and heart Zn concentrations increased with feeding biofortified wheat-containing 64 mg Zn/kg (P ≤ 0.002). Thigh meat Fe concentrations increased with increasing Zn concentrations of the wheat samples used (P = 0.0001), whereas the liver Cu concentrations decreased with feeding the wheat-containing 64 mg Zn/kg (P = 0.004). The Zn-biofortified wheat-containing greater Zn concentrations, particularly 64 mg Zn/kg, is a good replacement for corn in the poultry diet as long as its availability and low cost for better performance, greater digestibility, and elevated tissue Zn and Fe concentrations.
Similar content being viewed by others
Introduction
Zinc (Zn) is an essential trace element for humans and animals, including poultry. The importance of Zn in animal nutrition, particularly in poultry, comes from its necessity for growth (live performance), immunity, bone development, feathering, appetite, skin health, gene regulation, antioxidant defense, reproduction, and fast absorption [1,2,3,4]. Zinc has critical functions in immune response in the human body against various bacterial and viral attacks such as coronaviruses [5]. Zn also has particular structural and physiological processes in about 10% of the human proteins [6].
Cereals such as wheat, rice, and maize are relatively low in Zn contents (26–35 mg Zn/kg dry matter (DM) compared with animal protein sources [2]. The requirement of Zn for optimum poultry growth up to 21 days has been reported to be 40–50 mg Zn/kg DM [7, 8]. Therefore, a classical cereal-soybean meal-based diet of poultry requires a supplemental Zn. Dietary supplementation of Zn in poultry has been shown to provide better live performance and digestibility [9, 10].
Wheat (Triticum spp.) represents a significant part of the diet consumed in many countries, providing an ample proportion of energy and protein despite its low concentrations of minerals such as Zn and Fe [11, 12]. Therefore, Zn intake is restricted when a substantial part of the diet contains wheat which has a naturally low Zn concentration (20–40 mg Zn/kg DM) with poor bioavailability because of phytate-bound Zn, resulting in deficiency symptoms including retarded growth and depressed immunity in humans [13] as well as in poultry [14, 15].
Zinc deficiency is probably the most common micronutrient deficiency in arable soils. Although soils are sufficiently rich in Zn, the Zn in the soils is not chemically available for root absorption due to several adverse chemical and physical soil properties, including high soil pH, low organic matter, and low soil moisture [16]. Previously, it has been reported that about 50% of the cultivated soils globally have a low amount of plant-available Zn [17]. Consequently, plants grown on these soils contain a low amount of Zn in edible parts. Recently, Hill et al. [18] reported that grazing animals are at high risk of reduced Zn intake because the pastures contain very low Zn due to the low amount of plant-available Zn in soils. Today, there is an increasing trend to enrich (biofortify) pasture and fodder with Zn to ensure better Zn nutrition of animals. One strategy used for biofortification of pastures is agronomic biofortification (i.e., application of Zn fertilizers) [12].
Today agronomic biofortification of food and feed crops with micronutrients, especially Zn, is becoming very popular [16, 19, 20]. Compared to soil Zn application, foliar application of Zn fertilizers has been found to be highly effective in increasing grain Zn concentration [21]. In a recent human zinc absorption study, it has been shown that wheat biofortified with Zn by applying Zn had a distinct effect on dietary zinc absorption in humans, and this agronomic approach could help reduce the global burden of Zn deficiency [22].
Published reports show that among the interventions focusing on enrichment of foods or feeds with micronutrients, genetic biofortification (through classical breeding) and agronomic biofortification (through fertilizer applications) are similar or even more cost-effective than food fortification or supplementation for long term. In addition, there is also a high potential to improve the yield of plants in the field by applying micronutrient-containing fertilizers because soils are usually low in micronutrients, especially with Zn [16, 20, 23].
To our knowledge, the effect of agronomically biofortified wheat on the growth and development of poultry has not been studied. In the present study, we first produced wheat grains containing three different Zn concentrations by applying ZnSO4 to foliar. These Zn-biofortified wheat grains were then used to study their effects on performance, carcass characteristics, nutrient digestibility, and serum and tissue mineral concentrations in broiler-type quails.
Materials and Methods
Wheat Grain Material
In order to obtain wheat grains differing in Zn concentrations, bread wheat (Triticum aestivum) cultivar (Bezostaja) has been grown in Eskisehir, Central Anatolia in Turkey, as described by Cakmak et al. [21]. Wheat plants were sprayed with ZnSO4 either two times at the booting and milk stages or three times at the booting, anthesis, and milk stages. The control plots (no Zn application) were treated with water (see Cakmak et al. [21] for further details). The grains collected from the plots with different Zn applications were analyzed for Zn. The analysis results showed the following grain Zn results: (i) 18 mg Zn/kg (no Zn spray), (ii) 34 mg Zn/kg (2 times Zn spray), and (iii) 64 mg Zn/kg (3 times Zn spray). These grains were then used in the feeding trials described below.
Birds, Diets, Experimental Design
One hundred and eighty (one-day-old) Japanese quails (Coturnix coturnix japonica) were randomly divided into six equal groups, each containing 30 birds according to the body weights. Each group was subdivided into three replicates with ten birds. The birds were kept in cages with 20 × 20 cm dimensions, providing a space of 400 cm2 per bird in a temperature-controlled room at 24 ± 2 °C with 60 to 75% relative humidity.
The brooding temperature was 37.5 °C during the first week of life, with a weekly decline of 3 °C until room temperature (24–27 °C) was achieved. The photoperiod was 14 h light and 10 h dark (06.00–20.00 h). The birds were fed a starter diet until 21 days of age, followed by feeding a grower diet from day 21 to day 42 (Table 1). Feed and water were offered ad libitum throughout the experiment. The experiment was conducted in accordance with animal welfare regulations at the Veterinary Control and Research Institute of Elazig, Turkey (011/5–2).
All the diets were formulated based on the wheat–maize-soybean meal to meet or exceed NRC [24] requirements for quails and supplemented with the enzyme xylanase (200 mg/kg of feed; Ronozyme® WX (CT), also known as BioFeed® Wheat; DSM Nutritional Products, Kaiseraugst, Switzerland). The enzyme product contained a mono-component thermostable endo1,4–xylanase (EC 3.2.1.8) derived from Thermomyces lanuginosus, expressed in a genetically modified strain of Aspergillus oryzae. A coated dry product form of the enzyme was used with a minimum activity of 1000 FXU/g product (Farbe xylanase units).
The quails were fed regular diets including either (1) 18 mg Zn/kg DM-containing wheat along with no zinc picolinate (ZnPic) supplemented to the diet (W18), or (2) 18 mg Zn/kg DM-containing wheat along with 212.4 mg/kg ZnPic supplemented to the diet (W18 + Zn), or (3) 34 mg Zn/kg DM-containing wheat along with no ZnPic supplemented to the diet (W34), or (4) 34 mg Zn/kg DM-containing wheat along with 189.5 mg/kg ZnPic supplemented to the diet (W34 + Zn), or (5) 64 mg Zn/kg DM-containing wheat along with no ZnPic supplemented to the diet (W64), or (6) 64 mg Zn/kg DM-containing wheat along with 146.7 mg/kg ZnPic supplemented to the diet (W64 + Zn). In the zinc-supplemented groups, dietary zinc levels were adjusted to 50 mg Zn/kg of diet by supplementing an organic source of Zn as ZnPic based on recommended doses [3, 7, 8]. The concentrations of zinc in the ingredients and diets are shown in Table 2.
Data Collection
Cumulative feed intakes and body weight gains were recorded weekly, and weight gains and feed efficiencies were calculated. At the end of the study (day 42), 12 birds (two per replicate) randomly chosen from each treatment group were slaughtered for carcass evaluations and analyses of sera and tissues, namely liver, heart, and thigh meat. The birds were not fed before slaughter, and carcasses were yielded by removing feathers (wet), feet, and visceral organs. Blood samples (5 mL) were centrifuged at 3000 × g for 10 min for sera collection. The liver, heart, and left thigh were also removed for mineral analysis. The samples were stored at − 80 °C until analyses.
At the last 7 days of the experiment, 6 birds from each group were placed into individual battery cages for excreta collection to measure the digestibility of nitrogen, ash, Ca, P, Zn, Cu, and Fe. The excreta samples were oven-dried at 60 °C for 48 h and then ground to pass through a 40-mesh screen.
Laboratory Analyses
Feed and excreta samples were analyzed for crude protein (#988.05) and crude ash (#936.07) in triplicates [25]. Nutrient excretion (g/bird) was determined according to analyzed nutrient concentration in the excreta produced during the 7 days. Nutrient retention (g/bird) was calculated as nutrient intake (g/bird) minus nutrient excretion (g/bird). Nutrient retention (%) indicates the percentage of nutrients retained by the bird as a function of nutrient intake, and it was calculated as follows:
For detection of the concentrations of Ca, P, Zn, Cu, and Fe in feed, excreta, liver, heart, and thigh meat samples with 0.3 g each, along with 0.5 mL of serum samples, were weighed into a Teflon digestion vessel for digestion in 5 mL of 65% (v/v) spectra pure HNO3 (Suprapur, Merck, Darmstadt, Germany) in the model Speedwave™ MWS-2 Microwave Digestion System (Berghof, Eningen, Germany) which was equipped with a temperature, time, and power according to the digestion program. Sample solutions were diluted with 0.2% (v/v) nitric acid plus 0.2% (v/v) Triton X-100. Ca, Zn, Cu, and Fe concentrations were determined by flame atomic absorption spectrometry (F-AAS, AAS, Perkin-Elmer, Analyst 800, Norwalk, CT). The accuracy of quantitative measurements of Ca, Zn, Cu, and Fe was assured by simultaneous analysis of certified reference materials (NCS ZC730016 Chicken) which was digested analogously to the samples. The detection limits of Ca, Zn, Cu, and Fe were 45.0, 3.8, 1.5, and 0.3 µg/L, respectively. The mean recoveries of the reference material for tissues were as follows: Ca 95%, Fe 98%, Zn 95%, and Cu 99%.
Statistical Analyses
Data were analyzed by one-way ANOVA using SPSS, 2012 Version 21.0. In addition, post hoc Tukey procedure was run for multiple comparisons of the groups. The Shapiro–Wilk test was used to test the normality of the data. P < 0.05 was regarded statistically significant.
Results
Initial body weights of the quails were intended to be similar among treatments (P > 0.05). However, final body weights of the quails increased with the inclusion of greater Zn concentration of the biofortified-wheat samples in the diet, and additional ZnPic supplementation to each diet resulted in further but equal increases in final body weights (P = 0.0001; Fig. 1A). Bodyweight gains, as a reflection of the net body weight changes, were greater with the inclusion of the biofortified wheat-containing 64 mg Zn/kg DM to the diet compared with the other wheat samples (P = 0.0001), and supplementing ZnPic to each diet equally but further increased the body weight gains (P = 0.0001, Fig. 1B). The quails consumed more of the diet containing the wheat sample with 64 mg Zn/kg DM compared with other diets (P = 0.0001), and supplementing ZnPic to each diet equally but further increased feed intake (P = 0.0001, Fig. 1C). Feed efficiency expressed as feed intake over body weight gain (g/g) was greater with the inclusion of the wheat-containing 64 mg Zn/kg DM in the diet compared with the other wheat samples (P = 0.0001, Fig. 1D). Interestingly, when supplemented with an extra 1.6 g ZnPic per kg diet, feed efficiency remained similar for the diet containing the wheat-containing 64 mg Zn/kg DM (Fig. 1D). Unlike the inclusion of the wheat-containing 64 mg Zn/kg DM, however, supplementing ZnPic to the diets containing the other Zn-biofortified wheat samples with either 18 or 34 mg Zn/kg DM equally increased the feed efficiency (P = 0.0001). Nevertheless, all ZnPic-supplemented groups of quails had similar feed efficiency.
Final body weight (A), body weight gain (B), cumulative feed intake (C), feed efficiency: cumulative feed intake, g/body weight gain, g (D), cold carcass weight (E), cold carcass yield (F), liver weight (G) and heart weight(H) of quails fed different wheat varieties and supplemental organic Zn. Statistical comparisons are indicated with different superscript (a-c) located above the groups (p < 0.05; *ANOVA and Tukey’s post-hoc test)
Although the liver and heart weights of the quails were all similar among treatments (P > 0.05), cold carcass weights were greater with the inclusion of the wheat grains containing 64 mg Zn/kg DM to the diet compared with the other wheat grains (P = 0.0001, Fig. 1E–H), and supplementing ZnPic to each diet equally but further increased the cold carcass weights (P = 0.0001, Fig. 1E). However, all ZnPic-supplemented groups of quails had similar cold carcass weights. Cold carcass yields were similar among the quail-fed diets containing different wheat samples with different Zn but no supplementation. However, supplementing ZnPic to each diet increased the cold carcass yields, particularly the diet with the wheat-containing 34 mg Zn/kg DM (P = 0.001; Fig. 1F).
Nitrogen, Zn, and Fe retentions increased as the biofortified wheat samples had more Zn concentration (P ≤ 0.026; Table 3). Interestingly, when supplemented with an extra 1.6 g ZnPic per kg diet, the retentions remained similar for the diet containing the wheat sample with 64 mg Zn/kg DM. However, supplementing ZnPic to the diets containing other Zn biofortified wheat grains with either 18 or 34 mg Zn/kg DM equally increased the retentions. Nevertheless, all ZnPic-supplemented groups of quails had similar nitrogen, Zn, and Fe retentions. Ash and copper retentions changed similarly, such that inclusion of the Zn biofortified wheat grains containing 64 mg Zn/kg DM to the diet compared with other wheat samples provided greater retentions (P ≤ 0.026, Table 3) which were not further increased with an extra 1.6 g ZnPic per kg diet supplementation. Unlike the supplementation to the diet containing the wheat sample with 64 mg Zn/kg DM, supplementing ZnPic to the diets containing the other wheat samples with either 18 or 34 mg Zn/kg DM increased the retentions. Yet, ash and copper retentions remained similar in all ZnPic-supplemented groups of quails.
Calcium retention was greater with the inclusion of the wheat sample containing 64 mg Zn/kg to the diet compared with the other wheat grains with the lower amount of Zn (P = 0.003, Table 3), and supplementing ZnPic to each diet increased the retention, particularly with the diet having wheat samples with 64 mg Zn/kg DM, providing the greatest Ca retention. The diets containing the wheat samples with 34 and 64 mg Zn/kg DM yielded greater phosphorous retention compared with the diet containing the wheat grain with 18 mg Zn/kg (P = 0.014). However, supplementing ZnPic to each diet equally increased the P retentions bringing the values to similar ranges.
Excretion of nitrogen and ash was lower for the diets, including the biofortified wheat grains with 34 and 64 mg Zn/kg DM compared with wheat grains with 18 mg Zn/kg DM (P = 0.002, Table 3). The excretions equally decreased even more, when additional ZnPic was supplemented to each diet containing different wheat samples. Calcium excretion remained similar among diets containing different wheat samples having 18, 34, or 64 mg Zn/kg DM (P = 0.0001). However, supplementing ZnPic to each diet equally decreased Ca excretions. Inclusion of the wheat samples containing either 34 or 64 mg Zn/kg DM decreased the P excretion (P = 0.023), but supplementing ZnPic to all diets containing all wheat samples further but equally decreased the P excretion.
Inclusion of wheat varieties with increasing Zn concentration in the diet resulted in decreases in Zn and Cu excretion (P = 0.0001, Table 3), and supplementing ZnPic to each diet equally but further decreased Zn and Cu excretions, except for Cu excretion with the diet containing the wheat with 64 mg Zn/kg DM which remained unchanged when ZnPic was supplemented. Excretion of iron was lower for the diets, including the wheat samples with 34 and 64 mg Zn/kg DM compared with that of wheat with 18 mg Zn/kg (P = 0.001). The excretions were even lower when additional ZnPic was supplemented to the diets, including 18 or 64 mg/kg Zn-containing wheat samples compared with the 34 mg/kg Zn-containing wheat.
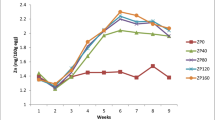
Changes in serum and tissue Zn, Cu, and Fe concentrations upon feeding different Zn biofortified-wheat samples and dietary ZnPic supplementation are shown in Fig. 2 and Table 4. Serum Zn concentrations equally increased when the wheat sample with 18 mg Zn/kg DM was replaced with the other wheat samples in the diet and with supplementation of ZnPic in each diet (P = 0.002, Fig. 2A). Liver and heart Zn concentrations increased with increasing Zn concentration of wheat samples (P = 0.001, Fig. 2B–C). The liver Zn concentrations further increased with each dietary supplementation of ZnPic. Although ZnPic supplementation resulted in further but equal increases in heart tissue, Zn concentrations of quails fed a diet containing the wheat samples with 18 and 34 mg Zn/kg DM compared with non-supplemental groups, supplementing ZnPic to the diet containing the wheat with 64 mg Zn/kg, had the greatest heart Zn concentrations. Thigh meat Zn concentrations increased, although not statistically significant, with increasing Zn concentration of the wheat samples. However, supplementing ZnPic to each diet equally increased the thigh meat Zn concentrations (P = 0.006, Fig. 2D), bringing the values to similar ranges within supplemented groups.
Serum and thigh meat Cu concentrations remained unchanged among treatments (P > 0.05, Table 4). The liver Cu concentrations decreased only with feeding the wheat-containing 64 mg Zn/kg DM (P = 0.004), the measured level of which was similar for all supplemental treatments. Inclusion of wheat samples with different Zn concentrations to the diets fed to quails decreased, although not significantly, the heart Cu concentrations. Supplementing ZnPic to the diets, particularly the wheat with the highest Zn, further reduced the heart Cu concentrations (P = 0.014).
Serum and heart Fe concentrations remained similar among treatments (P > 0.05, Table 4). Although not significantly, increasing Zn concentrations in the wheat grains resulted in increases in liver Fe concentrations. In addition, further increases with ZnPic supplementations were observed in liver Fe concentrations, particularly with ZnPic-supplemented wheat samples containing 64 mg Zn/kg DM as being greatest (P = 0.016). Thigh meat Fe concentrations increased with increasing Zn concentrations of the wheat (P = 0.0001). Further increases were observed with ZnPic supplementations to the diets, particularly with the diets containing wheat with 34 and 64 mg Zn/kg (P = 0.0001).
Discussion
Biofortification of food and feed crops with micronutrients is a growing interest and acceptance with particular positive effects. In a recent study, enrichment of silage maize with Zn, Se, and Fe by using fertilizers (i.e., agronomic biofortification) has been shown as a useful strategy to improve the micronutrient nutrition of animals [26]. The present study also showed that wheat enriched with Zn using agronomic biofortification is a highly useful strategy to improve animal feedstuffs with Zn.
Live performance, measured as body weight gains, feed intake, and feed efficiency, were greater when the quails were fed a diet containing wheat with 64 mg Zn/kg DM. Supplementing proper amounts of ZnPic to balance the required Zn levels in each diet provided similar live performances. Therefore, the inclusion of the Zn-biofortified wheat-containing 64 mg Zn/kg DM in the diet itself provides better performance, compared with those of other wheat samples, without any additional Zn sources. Similar results were also obtained from the cold carcass weights of the present work.
Deficiency of Zn in diets containing the wheat samples biofortified with lower levels of Zn (i.e., either 18 or 34 mg Zn/kg DM) without supplementation resulted in a slowed growth (less weight gain) and feed efficiency as compared with the wheat samples biofortified with Zn at the highest level (i.e., 64 mg Zn/kg DM) at the present work. The wheat samples containing 64 mg Zn/kg DM were close to the required level, thus providing the greatest live performance in quails. Retardation in growth and development as a result of Zn deficiency has been well-documented in poultry [27]. In ducks fed a Zn-deficient diet, significant decreases were found in growth, reproductive performance, and plasma antioxidant pool [28]. Zinc supplementation has also been proved to support chick growth, immune system, and feathering along with accelerating live performance and digestibility in poultry [4, 9, 10, 14]. However, contrary to the present work results, Yogesh et al. [29] reported no differences in growth parameters in broiler chicks fed a diet containing various Zn levels from 40 to 100 mg/kg DM. Similarly, the Zn level above 40 mg/kg DM has been shown not to influence the performance of broiler chicks [30, 31].
As far as carcass performance was concerned, the results from the present work were in agreement with the results from Yogesh et al. [29] and Rossi et al. [32], who reported unchanged carcass characteristics in chicks fed a diet with increasing Zn concentrations. Similarly, increasing the Zn supplement to the diet of Pekin ducks caused no changes in dressing percentages [33]. However, increases in carcass yields have been observed in broiler chicks fed a diet supplemented with either Zn-sulfate or Zn-methionine at 80 mg/kg DM [34]. The differences in growth conditions, the composition of feeding diets, and the amount and form of Zn added are probably responsible for such controversial results. For example, Olukosi et al. [35] showed that Zn hydroxychloride, compared with those of Zn sulfate, had better effects on growth parameters and meat yield in broiler chickens. In a further study, Olukosi et al. [36] found that using hydroxychloride trace minerals such as Zn, Cu, and Mn, compared with those of sulfate salts, in the diet of broilers and laying hens provided a better growth performance, reducing egg loss along with alleviated oxidative stress. Hydroxychloride trace minerals are released slowly due to their crystalline structure during digestion, resulting in better absorption and decreased excretion [37]. Similarly, Ao et al. [38] measured greater growth rates and bone Zn accumulation in broiler chicks fed a diet supplemented with organic Zn (Zn proteinate), as compared with inorganic Zn (ZnSO4). The supplemental Zn sources as ZnPic of the present work were used to minimize the interaction among other minerals and better bioavailability.
Improved digestibility of all nutrients (nitrogen, Ca, P, Zn, Cu, and Fe), measured as increased retentions and decreased excretions, were observed in quails fed a diet containing the wheat samples, particularly with 64 mg Zn/kg DM. Increased live performance of quails fed a diet with the wheat samples of 64 mg Zn/kg−1 DM was supported by the improved digestibility of the nutrients from the present work. However, contrary to the current work results, Yogesh et al. [29] found decreased Zn retentions with increasing Zn levels from 40 to 100 mg/kg DM in a diet fed to broiler chicks. Similarly, chicks were reported to excrete more Zn when the diet contained increasing Zn levels up to 178 mg/kg DM [39]. However, broiler chicks fed a diet supplemented with organic Zn (Zn-amino acid) compared with zinc sulfate up to 80 mg/kg DM were observed to excrete less Zn [40].
Increasing the Zn concentration of the wheat samples resulted in increases, as expected, in serum and tissue Zn concentrations at the present work. These results were also in agreement with the retention of Zn with increasing Zn concentration of the wheat samples. Increased tissue concentrations of Zn would be desirable in combating Zn deficiency upon consumption of these tissues by humans. The same is also true for consuming the wheat samples themselves containing 64 mg Zn/kg DM. Therefore, consuming either the wheat samples containing greater Zn concentration or the tissues (meat or organs) from the animals fed the same wheat samples offers a two-sided benefit. Similar to the present work results, supplemental organic or inorganic Zn sources to the diet of broiler chicks, laying hens, and Pekin ducks have been reported to increase blood and liver Zn concentrations [10, 14, 33]. Liver Zn concentrations are considered an important indicator of the whole-body level [41].
Since Zn is also involved in bone calcification in broilers, [42] increased Zn concentration in the diet was expected to increase Ca and P retentions, as evidenced in the present study. Although bone calcification was not measured at the current work, the increased levels of Zn in the diet have been reported to increase Zn and Ca concentrations in the tibia of laying hens [43], broiler chicks [34], and Pekin ducks [33].
Increasing the Zn concentration of the wheat samples also resulted in increases in liver and thigh Fe concentrations but decreases in liver and heart Cu concentrations at the present work. As opposed to the current study results, supplementing either organic or inorganic Zn to the diet of laying hens was found not to influence the blood Cu or Fe concentrations [10]. However, the antagonist features of Zn and Cu, as shown in the present work, were also found by Ognik et al. [44], who observed decreased Zn absorption from the intestines of chicks after Cu administrations with no effects on Fe absorption. Similar to the present work results, Olukosi et al. [35] also found that increasing Zn level in the diet of broiler chicks caused increases in plasma Zn but not in plasma Cu concentrations with no respect to Zn sources (sulfate or hydroxychloride) supplemented in the diet. However, in the same study [35], the liver Cu levels were greater with the inclusion of hydroxychloride Zn sources as compared to that of sulfate into the diet, independent from the Zn levels of the diet.
Although antagonistic features of Zn, Fe, and Cu have been known in chicks [45], the present work proved that the natural source of Zn in wheat does not interfere with Fe absorption or retention. Ao and Pierce [3] also indicated that the Zn, Fe, and Cu antagonisms could be avoided by supplementing organic Zn sources instead of inorganic ones in the poultry diet. Therefore, feeding the different wheat samples containing greater Zn concentration, particularly 64 mg Zn/kg DM, to quails increased both Zn and Fe concentrations of the animal tissues (meat and organs), which upon consumption offer health benefits to humans.
In general, supplementing ZnPic to balance the total Zn concentrations of the diets provided similar effects in responses to measured parameters at the present work. The main effect detected for the most measured parameters was the wheat samples, particularly one containing 64 mg Zn/kg DM. The bioavailability of Zn in wheat samples was made possible through enzyme inclusions in the diets.
Wheat as a palatable feed for poultry can be included in poultry diets up to 60% or more along with soybean meal. Wheat has about 94–96% of corn’s energy but greater protein, lysine, and tryptophan compared with those of corn [46]. Therefore, wheat is a good replacement for corn in the poultry diet due to its availability and low cost. This is also true for the wheat samples containing greater Zn concentrations. Although balanced and made ideal for human consumption, raising the Zn concentration of the wheat samples further, through biofortification, up to 50 mg/kg DM at the required level for the quail diets without compromising the balance of other nutrients needs be elucidated. Wheat, mostly used for human consumption, may not be appropriate for feeding animals due to its hardness and probably its starch type and concentration influencing the foregut digestibility of poultry, besides the cost issue. However, because of enzyme addition to the diet and limited inclusion (30%) of the wheat in the diet, these issues seem not a problem for feeding quails as evidenced at the present work, providing better performance, greater digestibility, and elevated serum and tissue Zn and Fe concentrations particularly with the wheat samples containing 64 mg Zn/kg DM. Although not meant to be designed particularly for animal consumption, the wheat samples with 64 mg Zn/kg DM are also a good choice as an animal feed, particularly for poultry.
Data Availability
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Park SY, Birkhold SG, Kubena LF, Nisbet DJ, Ricke SC (2004) Review on the role of dietary zinc in poultry nutrition, immunity, and reproduction. Biol Trace Elem Res 101(2):147–163. https://doi.org/10.1385/BTER:101:2:147
Suttle NF (2010) Mineral nutrition of livestock, 4th edn. CABI, Cambridge
Ao T, Pierce J (2013) The replacement of inorganic mineral salts with mineral proteinates in poultry diets. Worlds Poult Sci J 69(1):5–16. https://doi.org/10.1017/S0043933913000019
Huang L, Li X, Wang W, Yang L, Zhu Y (2019) The role of zinc in poultry breeder and hen nutrition: an update. Biol Trace Elem Res 192(2):308–318. https://doi.org/10.1007/s12011-019-1659-0
Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G (2019) The role of zinc in antiviral ımmunity. Adv Nutr 10(4):696–710. https://doi.org/10.1093/advances/nmz013
Krężel A, Maret W (2016) The biological inorganic chemistry of zinc ions. Arch Biochem Biophys 611:3–19. https://doi.org/10.1016/j.abb.2016.04.010
Huang YL, Lu L, Luo XG, Liu B (2007) An optimal dietary zinc level of broiler chicks fed a corn-soybean meal diet. Poult Sci 86:2582–2589. https://doi.org/10.3382/ps.2007-00088
Linares LB, Broomhead JN, Guaiume EA, Ledoux DR, Veum TL, Raboy V (2007) Effects of low phytate barley (Hordeum vulgare L.) on zinc utilization in young broiler chicks. Poult Sci 86(12):299–308
Huang YL, Lu L, Li SF, Luo XG, Liu B (2009) Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed a conventional corn-soybean meal diet. J Anim Sci 87(6):2038–2046. https://doi.org/10.2527/jas.2008-1212
Abedini M, Shariatmadari F, Karimi Torshizi MA, Ahmadi H (2018) Effects of zinc oxide nanoparticles on the egg quality, immune response, zinc retention, and blood parameters of laying hens in the late phase of production. J Anim Physiol Anim Nutr (Berl) 102(3):736–745. https://doi.org/10.1111/jpn.12871
Gibson RS (2007) The role of diet- and host-related factors in nutrient bioavailability and thus in nutrient-based dietary requirement estimates. Food Nutr Bull 28(1 Suppl International):S77–100. https://doi.org/10.1177/15648265070281S108
Cakmak I, Kutman UB (2018) Agronomic biofortification of cereals with zinc: a review. Eur J Soil Sci 69(1):172–180. https://doi.org/10.1111/ejss.12437
Welch RM, Graham RD (2004) Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55:353–364. https://doi.org/10.1093/jxb/erh064
Ao T, Pierce JL, Pescatore AJ, Cantor AH, Dawson KA, Ford MJ, Paul M (2011) Effects of feeding different concentration and forms of zinc on the performance and tissue mineral status of broiler chicks. Br Poult Sci 52(4):466–471. https://doi.org/10.1080/00071668.2011.588198
Naz S, Idris M, Khalique MA, Zia-Ur-Rahman AIA, Abdelrahman MM, Khan RU, Chand N, Farooq U, Ahmad S (2016) The activity and use of zinc in poultry diets. Worlds Poult Sci J 72(1):159–167. https://doi.org/10.1017/S0043933915002755
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302(1–2):1–17. https://doi.org/10.1007/s11104-007-9466-3
Graham RD, Welch RM, Bouis HE (2001) Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Adv Agron 70:77–142. https://doi.org/10.1016/S0065-2113(01)70004-1
Hill GM, Shannon MC (2019) Copper and zinc nutritional ıssues for agricultural animal production. Biol Trace Elem Res 188(1):148–159. https://doi.org/10.1007/s12011-018-1578-5
Zou C, Du Y, Rashid A, Ram H, Savasli E, Pieterse PJ, Ortiz-Monasterio I, Yazici A, Kaur C, Mahmood K, Singh S, Le Roux MR, Kuang W, Onder O, Kalayci M, Cakmak I (2019) Simultaneous biofortification of wheat with zinc, iodine, selenium, and iron through foliar treatment of a micronutrient cocktail in six countries. J Agric Food Chem 67(29):8096–8106. https://doi.org/10.1021/acs.jafc.9b01829
Garg M, Sharma N, Sharma S, Kapoor P, Kumar A, Chunduri V, Arora P (2018) Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front Nutr 5:12. https://doi.org/10.3389/fnut.2018.00012
Cakmak I, Kalayci M, Kaya Y, Torun AA, Aydin N, Wang Y, Arisoy Z, Erdem H, Yazici A, Gokmen O, Ozturk L, Horst WJ (2010) Biofortification and localization of zinc in wheat grain. J Agric Food Chem 58(16):9092–9102. https://doi.org/10.1021/jf101197h
Signorell C, Zimmermann MB, Cakmak I, Wegmüller R, Zeder C, Hurrell R, Aciksoz SB, Boy E, Tay F, Frossard E, Moretti D (2019) Zinc absorption from agronomically biofortified wheat is similar to post-harvest fortified wheat and is a substantial source of bioavailable zinc in humans. J Nutr 149(5):840–846. https://doi.org/10.1093/jn/nxy328
Joy EJM, Ahmad W, Zia MH, Kumssa DB, Young SD, Ander EL, Watts MJ, Stein AJ, Broadley MR (2017) Valuing increased zinc (Zn) fertiliser-use in Pakistan. Plant Soil 411(1):139–150. https://doi.org/10.1007/s11104-016-2961-7
Council NR (1994) Nutrient requirements of poultry, 9th revise. The National Academies Press, Washington, DC
A.O.A.C. (1990) Official Methods of Analysis. 15th Edition, Association of Official Analytical Chemist, Washington DC
Grujcic D, Yazici AM, Tutus Y, Cakmak I, Singh BR (2021) Biofortification of silage maize with zinc, iron and selenium as affected by nitrogen fertilization. Plants 10(2):1–13. https://doi.org/10.3390/plants10020391
Salim HM, Jo C, Lee BD (2008) Zinc in broiler feeding and nutrition. Avian Biol Res 1(1):5–18. https://doi.org/10.3184/175815508X334578
Zhang YN, Wang S, Li KC, Ruan D, Chen W, Xia WG, Wang SL, Abouelezz KFM, Zheng CT (2020) Estimation of dietary zinc requirement for laying duck breeders: effects on productive and reproductive performance, egg quality, tibial characteristics, plasma biochemical and antioxidant indices, and zinc deposition. Poult Sci 99(1):454–462. https://doi.org/10.3382/ps/pez530
Yogesh K, Deo C, Shrivastava HP, Mandal AB, Wadhwa A, Singh I (2013) Growth performance, carcass yield, and ımmune competence of broiler chickens as ınfluenced by dietary supplemental zinc sources and levels. Agric Res 2(3):270–274. https://doi.org/10.1007/s40003-013-0067-5
Mohanna G, Nys Y (1999) Effect of dietary zinc content and sources on the growth, body zinc deposition and retention, zinc excretion and immune response in chickens. Br Poult Sci 40(1):108–114. https://doi.org/10.1080/00071669987926
Collins NE, Moran ET (1999) Influence of supplemental manganese and zinc on live performance and carcass quality of broilers. J Appl Poult Res 8(2):222–227. https://doi.org/10.1093/japr/8.2.222
Rossi P, Rutz F, Anciuti MA, Rech JL, Zauk NHF (2007) Influence of graded levels of organic zinc on growth performance and carcass traits of broilers. J Appl Poult Res 16(2):219–225. https://doi.org/10.1093/japr/16.2.219
Wen M, Wu B, Zhao H, Liu G, Chen X, Tian G, Cai J, Jia G (2019) Effects of dietary zinc on carcass traits, meat quality, antioxidant status, and tissue zinc accumulation of pekin ducks. Biol Trace Elem Res 190(1):187–196. https://doi.org/10.1007/s12011-018-1534-4
Mohammadi V, Ghazanfari S, Mohammadi-Sangcheshmeh A, Nazaran MH (2015) Comparative effects of zinc-nano complexes, zinc-sulphate and zinc-methionine on performance in broiler chickens. Br Poult Sci 56(4):486–493. https://doi.org/10.1080/00071668.2015.1064093
Olukosi OA, Van Kuijk S, Han Y (2018) Copper and zinc sources and levels of zinc inclusion influence growth performance, tissue trace mineral content, and carcass yield of broiler chickens. Poult Sci 97(11):3891–3898. https://doi.org/10.3382/ps/pey247
Olukosi OA, Van Kuijk SJA, Han Y (2019) Sulfate and hydroxychloride trace minerals in poultry diets - comparative effects on egg production and quality in laying hens, and growth performance and oxidative stress response in broilers. Poult Sci 98(10):4961–4971. https://doi.org/10.3382/ps/pez261
Hawthorne FC, Sokolova E (2002) Simonkolleite, Zn5 (OH)8 Cl2 (H2O), a decorated interrupted-sheet structure of the form [MΦ2]4. Can Mineral 40(3):939–946. https://doi.org/10.3749/gscanmin.40.3.939
Ao T, Pierce JL, Power R et al (2006) Evaluation of Bioplex Zn® as an organic zinc source for chicks. Int J Poult Sci 5(9):808–811. https://doi.org/10.3923/ijps.2006.808.811
Stahl JL, Cook ME, Sunde ML, Gregor JL (1989) Enhanced humoral immunity in progeny chicks from hens fed practical diets supplemented with zinc. Appl Agric Res 4:86–89
Burrell AL, Dozier WA, Davis AJ, Compton MM, Freeman ME, Vendrell PF, Ward TL (2004) Responses of broilers to dietary zinc concentrations and sources in relation to environmental implications. Br Poult Sci 45(2):225–263. https://doi.org/10.1080/00071660410001715867
Jahanian R, Rasouli E (2015) Effects of dietary substitution of zinc-methionine for inorganic zinc sources on growth performance, tissue zinc accumulation and some blood parameters in broiler chicks. J Anim Physiol Anim Nutr (Berl) 99(1):50–58. https://doi.org/10.1111/jpn.12213
Star L, van der Klis JD, Rapp C, Ward TL (2012) Bioavailability of organic and inorganic zinc sources in male broilers. Poult Sci 91(12):3115–3120. https://doi.org/10.3382/ps.2012-02314
Min YN, Liu FX, Qi X, Ji S, Cui L, Wang ZP, Gao YP (2019) Effects of organic zinc on tibia quality, mineral deposit, and metallothionein expression level of aged hens. Poult Sci 98(1):366–372. https://doi.org/10.3382/ps/pey386
Ognik K, Stȩpniowska A, Cholewińska E, Kozłowski K (2016) The effect of administration of copper nanoparticles to chickens in drinking water on estimated intestinal absorption of iron, zinc, and calcium. Poult Sci 95(9):2045–2051. https://doi.org/10.3382/ps/pew200
Rama R, Planas J (1981) Dietary cadmium effect on iron metabolism in chickens. Biol Trace Elem Res 3(3):169–183. https://doi.org/10.1007/BF02990115
McNab JM, Boorman KN (2002) Poultry feedstuffs: supply, composition and nutritive value. CABI Publishing Wallingford, UK. https://doi.org/10.1079/9780851994642.0000
Funding
This work was supported by the Turkish Academy of Sciences partly (KS).
Author information
Authors and Affiliations
Contributions
Conceptualization, K.S. and I.C.; data curation, N.S. and C.O., methodology, N.S., O.K., C.O., and I.C.; formal analysis, N.S., E.S., C.O., K.S., and I.C.; writing—original draft preparation, O.K. and K.S.; and writing—review and editing, K.S. and I.C. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approvals
The experiment was conducted in accordance with animal welfare regulations at the Veterinary Control and Research Institute of Elazig, Turkey.
Consent to Participate
All participants provided written informed consent before participating in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahin, N., Kucuk, O., Orhan, C. et al. Feeding Zinc-Biofortified Wheat Improves Performance, Nutrient Digestibility, and Concentrations of Blood and Tissue Minerals in Quails. Biol Trace Elem Res 200, 3774–3784 (2022). https://doi.org/10.1007/s12011-021-02955-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02955-0