Abstract
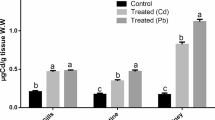
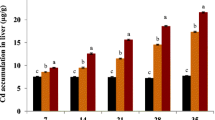
The current study aims to assess the cadmium sub-lethal concentration influence on growth and haematological and biochemical parameters of Mystus seenghala. A total of 60 fish of three different length groups (20 each) were collected from Head Qadirabad, Pakistan. The fish were treated to the sub-lethal concentration viz. one-third of LC50, for 16 weeks except for the control groups. Water quality parameters were kept constant during the entire course of the research, and the major parameters were measured as temperature (28.03 ± 0.03 °C), DO (5.82 ± 0.14 mg L−1), pH (8.00 ± 0.01) and total hardness (249.98 ± 0.01 mg L−1). Findings revealed that the growth of three treated variant length groups was affected negatively by cadmium exposure and showed significantly (P < 0.05) lower average wet weight, body length and condition factor as compared to control groups, while the feed conversion ratio (FCR) increases by increasing the exposure duration. The haematological parameters including values of Hct, Hb and MCHC were significantly (P < 0.05) reduced in all Cd-treated groups than control groups, whereas the level of MCH and MCV were significantly higher, but no significant difference was found in the value of RBCs in all treated groups. Biochemical parameters such as ALT, AST, total lipid and glucose level in Cd exposure groups were significantly higher, while the total protein level was significantly (P < 0.05) reduced in all treated groups as compared to control groups. From the current study, it has been concluded that the growth, haematology and biochemical parameters are important indicators of ecotoxicology particularly contamination of the cadmium and health of the fish.




Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Ghiasi F, Mirzargar SS, Badakhshan H, Shamsi S (2010) Effects of low concentration of cadmium on the level of lysozyme in serum, leukocyte count and phagocytic index in Cyprinus carpio under the wintering conditions. J Fish Aquat Sci 5:113–119. https://doi.org/10.3923/jfas.2010.113.119
Paul D, Sinha SN (2013) Assessment of various heavy metals in surface water of polluted sites in the lower stretch of river Ganga, West Bengal: a study for ecological impact. Discov Nat 6:8–13
Ashraf MA, Maah MJ, Yusoff I, Mehmood K (2010) Effects of polluted water irrigation on environment and health of people in Jamber, District Kasur, Pakistan. Int J Sci Basic Appl 10:37–57
Gaber HS, El-Kasheif MA, Ibrahim SA, Authman M (2013) Effect of water pollution in El-Rahawy drainage canal on hematology and organs of freshwater fish. World Appl Sci J 21:329–341. https://doi.org/10.5829/idosi.wasj.2013.21.3.71192
Medeiros RJ, dos Santos LMG, Freire AS, Santelli RE, Braga AMC, Krauss TM, Jacob SDC (2012) Determination of inorganic trace elements in edible marine fish from Rio de Janeiro State, Brazil. Food Control 23:535–541. https://doi.org/10.1016/j.foodcont.2011.08.027
Meche A, Martins MC, Lofrano BE, Hardaway CJ, Merchant M, Verdade L (2010) Determination of heavy metals by inductively coupled plasma-optical emission spectrometry in fish from the Piracicaba River in Southern Brazil. Microchem J 94:171–174. https://doi.org/10.1016/j.microc.2009.10.018
Kerambrun E, Henry F, Perrichon P, Courcot L, Meziane T, Spilmont N, Amara R (2012) Growth and condition indices of juvenile turbot, Scophthalmus maximus, exposed to contaminated sediments: effects of metallic and organic compounds. Aquat toxicol 108:130–140. https://doi.org/10.1016/j.aquatox.2011.07.016
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture. Aquaculture 11:8–15. https://doi.org/10.1016/j.aquaculture.2018.10.030
Habib SS, Naz S, Nawaz S, Ameer I, Khatoon A, Rehman HU, Jawad SM, Ali H (2021) Comparative analysis of hematological parameters of some farmed and wild fish species. Pak J Zool. https://doi.org/10.17582/journal.pjz/20200124050118
Jayaram KC (1999) The freshwater fishes of the Indian region. Narendra Publishing House, Delhi
Shafri M, Abdul Manan MJ (2012) Therapeutic potential of the haruan (Channa striatus): from food to medicinal uses. Malays J Nutr 18:125–136
Javed M (2012) Growth responses of fish under chronic exposure of waterborne and dietary metals. Int J Agric Biol 14:281–285
APHA AWWA (2012) WEF. (2012). Standard methods for the examination of water and wastewater, 22.
Kesbiç OS (2019) Effects of juniper berry oil on growth performance and blood parameters in common carp (Cyprinus carpio). Aquac Res 50:342–349. https://doi.org/10.1111/are.13908
Schaperclaus W, Kulow H, Schreckenbach K (1991) Hematological and serological technique. Oxonian Press, New Delhi, India
Goldenfarb PB, Bowyer FP, Hall T, Brosious E (1971) Reproducibility in the hematology laboratory: the microhematocrit determination. Am J Clin Pathol 56:35–39. https://doi.org/10.1093/ajcp/56.1.35
Britton CJ, Whitby LE (1963) Disorders of the blood: diagnosis, pathology, treatment, technique. With a Chapter on 'The Cytochemistry of Haemopoiesis' by FG Hayhoe and a Chapter on 'Blood Groups and Blood Transfusion' by Geoffrey H. Tovey. Churchill.
Landis WG, Yu M (2004) Introduction to environmental toxicology. CRC Press
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27. https://doi.org/10.1177/000456326900600108
Henry RJ (1964) Colorimetric determination of total protein: clinical chemistry. Harper and Row, New York
Knight JA, Anderson S, Rawle JM (1972) Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin chem 18:199–202. https://doi.org/10.1093/clinchem/18.3.199
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63. https://doi.org/10.1093/ajcp/28.1.56
McRae NK, Gaw S, Glover CN (2018) Effects of waterborne cadmium on metabolic rate, oxidative stress, and ion regulation in the freshwater fish, inanga (Galaxias maculatus). Aquat Toxicol 194:1–9. https://doi.org/10.1016/j.aquatox.2017.10.027
Almeida JA, Novelli ELB, Silva MDP, Júnior RA (2001) Environmental cadmium exposure and metabolic responses of the Nile tilapia, Oreochromis niloticus. Environ Pollut 114:169–175. https://doi.org/10.1016/S0269-7491(00)00221-9
Paul JS, Small BC (2021) Chronic exposure to environmental cadmium affects growth and survival, cellular stress, and glucose metabolism in juvenile channel catfish (Ictalurus punctatus). Aquat Toxicol 230:105705
Javed M (2015) Chronic dual exposure (waterborne+ dietary) effects of cadmium, zinc and copper on growth and their bioaccumulation in Cirrhina mrigala. Pak Vet J 35:143–146
Ayyat MS, Mahmoud HK, El-Hais AEAM, Abd El-Latif KM (2017) The role of some feed additives in fish fed on diets contaminated with cadmium. Environ Sci Pollut Res 24:23636–23645. https://doi.org/10.1007/s11356-017-9986-1
Jezierska B, Ługowska K, Witeska M (2008) The effects of heavy metals on embryonic development of fish (a review). Fish Physiol Biochem 35:625–640. https://doi.org/10.1007/s10695-008-9284-4
Ansari RA, Kaur M, Ahmad F, Rahman S, Rashid H, Islam F, Raisuddin S (2009) Genotoxic and oxidative stress-inducing effects of deltamethrin in the erythrocytes of a freshwater biomarker fish species, Channa punctata Bloch. Environ Toxicol: Int J 24:429–436. https://doi.org/10.1002/tox.20445
Tsai JW, Liao CM (2006) Mode of action and growth toxicity of arsenic to tilapia Oreochromis mossambicus can be determined bioenergetically. Arch Environ Contam Toxicol 50:144–152. https://doi.org/10.1007/s00244-005-1054-z
Jabeen G, Javed M, Azmat H (2012) Assessment of heavy metals in the fish collected from the river Ravi, Pakistan. Pak Vet J 32:107–111
Mekkawy IA, Mahmoud UM, Wassif ET, Naguib M (2011) Effects of cadmium on some haematological and biochemical characteristics of Oreochromis niloticus (Linnaeus, 1758) dietary supplemented with tomato paste and vitamin E. Fish Physiol Biochem 37:71–84. https://doi.org/10.1007/s10695-010-9418-3
Debasmita S, Sahu S, Singh A, Mohapatra AK (2016) Hematotoxic effects of cadmium on fresh water cat fish, Clarias gariepinus (Burchell, 1822). Environ Monit Assess 187:172–187
Sharma J, Langer S (2014) Effect of manganese on haematological parameters of fish, Garra gotyla gotyla. J Entomol Zool Stud 2:77–81
Shalaby AME (2007) Effect of EDTA on toxicity reduction of cadmium in relation to growth, some haematological and biochemical profiles of Nile tilapia (Oreochromis niloticus). J Fish Aquatic Sci 2:100–109. https://doi.org/10.3923/jfas.2007.100.109
El-Boshy MES, Gadalla HA, El-Hamied FMA (2014) Immunological, hematological and biochemical changes induced by short term exposure to cadmium in catfish (Clarias gariepinus). J Coast Life Med 2:175–180. https://doi.org/10.12980/JCLM.2.2014J57
Fawole FJ, Adeoye AA, Tiamiyu LO, Ajala KI, Obadara SO, Ganiyu IO (2020) Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): effects on growth, nutrient utilization, haemato-physiological response, and oxidative stress biomarker. Aquaculture 518:734849
Svoboda M (2001) Stress in fish–review. Bul VURH Vodnany 37:69–191
Chen C, Wooster GA, Bowser PR (2004) Comparative blood chemistry and histopathology of tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin or copper sulfate. Aquaculture 239:421–443. https://doi.org/10.1016/j.aquaculture.2004.05.033
Kumar K (2016) A review of heavy metals toxicological studies on mangur (Clarias batrachus) fish. Int J Eng Manag Res 6:577–583
Shalaby AME (2001) Protective effect of ascorbic acid against mercury intoxication in Nile tilapia (Oreochromius niloticus). J Egypt Acad Soc Environ Develop (D-Environ Study) 2:79–97
Makaras T, Razumienė J, Gurevičienė V, Šakinytė I, Stankevičiūtė M, Kazlauskienė N (2020) A new approach of stress evaluation in fish using β-d-glucose measurement in fish holding-water. Ecol Indic 109:105829. https://doi.org/10.1016/j.ecolind.2019.105829
Islam SM, Rohani MF, Zabed SA, Islam MT, Jannat R, Akter Y, Shahjahan M (2020) Acute effects of chromium on hemato-biochemical parameters and morphology of erythrocytes in striped catfish Pangasianodon hypophthalmus. Toxicol Rep 7:664–670. https://doi.org/10.1016/j.toxrep.2020.04.016
Moniruzzaman M, Kumar S, Das D, Sarbajna A, Chakraborty SB (2020) Enzymatic, non enzymatic antioxidants and glucose metabolism enzymes response differently against metal stress in muscles of three fish species depending on different feeding niche. Ecotoxicol Environ Saf 202:110954. https://doi.org/10.1016/j.ecoenv.2020.110954
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All experimental trials were carried out under European legislation concerning the protection of animals used for scientific purposes (European Directive 2010/63), and the trial was registered with the University of Mianwali Fisheries Department under trial number UM/F/MS-112.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fazio, F., Habib, S.S., Naz, S. et al. Cadmium Sub-Lethal Concentration Effect on Growth, Haematological and Biochemical Parameters of Mystus seenghala (Sykes, 1839). Biol Trace Elem Res 200, 2432–2438 (2022). https://doi.org/10.1007/s12011-021-02852-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02852-6