Abstract
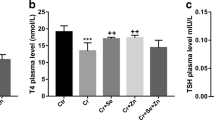
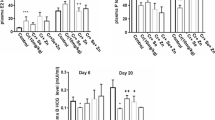
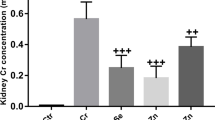
Nickel chloride (NiCl2) is a heavy metal that may affect the function of the thyroid. Selenium (Se) and zinc (Zn) are essential trace elements involved in thyroid hormone metabolism. However, little is reported about thyrotoxicity during gestation. The current study aimed to investigate the protective effects of selenium and zinc against NiCl2-induced thyrotoxicity in pregnant Wistar rats. Female rats were treated subcutaneously (s.c.) on the 3rd day of pregnancy, with NaCl 0.9% and served as control, NiCl2 (100 mg/kg body weight (BW)) alone, or in association with Se (0.3 mg/kg, s.c.), ZnCl2 (20 mg/kg, s.c.), or both of them simultaneously. Oxidative stress parameters, thyroid biomarkers, and histopathological examination were evaluated. Results showed that NiCl2 exposure caused a significant decrease in maternal body weight and an increase in absolute and relative thyroid weight compared to the controls. NiCl2 administration also led to decreased plasma triiodothyronine (T3) and thyroxine (T4) with a concomitant significant increase in thyroid-stimulating hormone (TSH) levels when compared to that of control. In addition, an overall pro-oxidant effect was associated with a decrease in the reduced glutathione (GSH) and nonprotein thiol (NPSH) contents and the enzymatic activity of glutathione peroxidase (GPx) and superoxide dismutase (SOD), and an increase in malondialdehyde (MDA). These biochemical disturbances were confirmed by histological changes. However, the co-treatment of Se and/or ZnCl2 attenuates NiCl2-induced changes. Our findings suggested that Se and ZnCl2 ameliorated NiCl2-induced thyrotoxicity in pregnant Wistar rats by exhibiting antioxidant effects.


Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon request.
Code Availability
Not applicable.
References
Brevini L, Tiziana A, Zanetto SB, Cillo F (2005) Effects of endocrine disruptors on developmental and reproductive functions. Curr Drug Targets-Immune Endocr Metab Disord 5(1):1–10. https://doi.org/10.2174/1568008053174750
Tebourbi O, Hallegue D, Yacoubi MT, Sakly M, Rhouma KB (2010) Subacute toxicity of p, p′-DDT on rat thyroid: hormonal and histopathological changes. Environ Toxicol Pharmacol 29(3):271–279. https://doi.org/10.1016/j.etap.2010.03.002
Wang X, Ouyang F, Feng L, Wang X, Liu Z, Zhang J (2017) Maternal urinary triclosan concentration in relation to maternal and neonatal thyroid hormone levels: a prospective study. Environ Health Perspect 125(6):067017. https://doi.org/10.1289/EHP500
Morreale DEG, Obregon MJ, Escobar DRF (2000) Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 85(11):3975–3987. https://doi.org/10.1210/jcem.85.11.6961
Morreale DEG, Obregon MJ, Escobar DRF (2004) Role of thyroid hormone during early brain development. Eur J Endocrinol 151(3):U25–U37
Zambelli B, Uversky VN, Ciurli S (2016) Nickel impact on human health an intrinsic disorder perspective. Biochim Biophys Acta1 864(12):1714–31
Ijomone OM, Olatunji SY, Owolabi JO, Naicker T, Aschner M (2018) Nickel-induced neurodegeneration in the hippocampus, striatum and cortex; an ultrastructural insight, and the role of caspase-3 and α-synuclein. J Trace Elem Med Biol 50:16–23. https://doi.org/10.1016/j.jtemb.2018.05.017
Keinan D, Mass E, Zilberman U (2010) Absorption of nickel, chromium, and iron by the root surface of primary molars covered with stainless steel crowns. Int J Dent 2010. https://doi.org/10.1155/2010/326124
Zeinali T, Salmani F, Naseri K (2019) Dietary intake of cadmium, chromium, copper, nickel, and lead through the consumption of meat, liver, and kidney and assessment of human health risk in Birjand, southeast of Iran. Biol Trace Elem Res 191(2):338–347. https://doi.org/10.1007/s12011019.1637.6
Minigaliyeva IA, Katsnelson BA, Privalova LI et al (2014) Toxicodynamic and toxicokinetic descriptors of combined chromium (VI) and nickel toxicity. Int J Toxicol 33(6):498–505. https://doi.org/10.1177/1091581814555915
Das KK, Buchner V (2007) Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health 22(2):157. https://doi.org/10.1515/reveh.2007.22.2.157
Lushchak VI (2011) Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Physiol C Toxicol Pharmacol 153(2):175–190. https://doi.org/10.1016/j.cbpc.2010.10.004
Pari L, Prasath A (2008) Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chem Biol Interact 173(2):77–83. https://doi.org/10.1016/j.cbi.2008.02.010
Ijomone OM, Okori SO, Ijomone OK, Ebokaiwe AP (2018) Sub-acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats brain. Drug Chem Toxicol 41(4):377–384. https://doi.org/10.1080/01480545.2018.1437173
Xu S, He M, Zhong M et al (2015) The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neurosci Lett 590:52–57. https://doi.org/10.1016/j.neulet.2015.01.065
Goodman JE, Prueitt RL, Dodge DG, Thakali S (2009) Carcinogenicity assessment of watersoluble nickel compounds. Crit Rev Toxicol 39(5):365–417. https://doi.org/10.1080/10408440902762777
Adjroud O, Mouffok S (2009) Effects of nickel chloride on hematological and developmental parameters in Wistar albino pregnant rats. Ass Univ Bull Environ Res 12(1):1–9. https://doi.org/10.21608/AUBER.2009.149529
Mahmoodianfard S, Vafa M, Golgiri F, Khoshniat M, Gohari M, Solati Z, Djalali M (2015) Effects of zinc and selenium supplementation on thyroid function in overweight and obese hypothyroid female patients: a randomized double-blind controlled trial. J Am Coll Nutr 34(5):391–399. https://doi.org/10.1080/07315724.2014.926161
Wiersinga WM (2016) Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease. Endocrinol Metab 31(2):213. https://doi.org/10.3803/EnM.2016.31.2.213
Berry MJ, Banu L, Larsen PR (1991) Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 349(6308):438–440. https://doi.org/10.1038/349438a0
Schomburg L (2011) Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol 8(3):160–171. https://doi.org/10.1038/nrendo.2011.174
Pelyhe C, Mézes M (2013) Myths and facts about the effects of nano selenium in farm animals-mini-review. Eur Chem Bull 2(12):1049–1052
Käkelä R, Käkelä A, Hyvärinen H (1999) Effects of nickel chloride on reproduction of the rat and possible antagonistic role of selenium. Comp Biochem Physiol C: Pharmacol. Toxicol and Endocrinol 123(1):27–37. https://doi.org/10.1016/S0742-8413(99)00006-7
Adjroud O (2013) The toxic effects of nickel chloride on liver, erythropoiesis, and development in Wistar albino preimplanted rats can be reversed with selenium pretreatment. Environ Toxicol 28(5):290–298. https://doi.org/10.1002/tox.20719
Formigari A, Irato P, Santon A (2007) Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Phys C 146(4):443–459. https://doi.org/10.1016/j.cbpc.2007.07.010
Catania AS, Barros CR, Ferreira SRG (2009) Vitamins and minerals with antioxidant properties and cardiometabolic risk: controversies and perspectives. Arq Bras Endocrinol Metabol 53(5):550–559
Severo JS, Morais JBS, de Freitas TEC, Andrade ALP, Feitosa MM, Fontenelle LC, de Oliveira ARS et al (2019) The role of zinc in thyroid hormones metabolism. Int J Vitamin Nut Res 89:80–88. https://doi.org/10.1024/0300-9831/a000262
Paksy K, Varga B, Lázár P (1997) Zinc protection against cadmium induced infertility in female rats. Effect of zinc and cadmium on the progesterone production of cultured granulosa cells. BioMetals 10:27–36. https://doi.org/10.1023/A:1018362603065
Ghabaee DNZ, Amiri FT, Moghaddam AE, Khalatbary AR, Zargari M (2017) Administration of zinc against arsenic-induced nephrotoxicity during gestation and lactation in rat model. J Nephropathol 6(2):74–80. https://doi.org/10.15171/jnp.2017.13
Lowry OH, Rosebrugh NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-I
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzeneoxide as the hepatotoxic intermediate. Pharmacology 11(3):151–169. https://doi.org/10.1159/000136485
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161(2):559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Meth enzymol 105 :114–121
Liang C, Han Y, Ma L et al (2020) Low levels of arsenic exposure during pregnancy and maternal and neonatal thyroid hormone parameters: the determinants for these associations. Environ Int 145:106114. https://doi.org/10.1016/j.envint.2020.106114
Cheng WZ, Yin CF (1992) Effects of nickel chloride (NiCl2) and nickel sulfate (NiSO4) on T3, T4, TSH content in rat serum. J. Shijiazhuang Med Coll 3:4–7
Liu YH, Chen H, Zhang L, Zhang T, Ren X (2019) The association between thyroid injury and apoptosis, and alterations of Bax, Bcl-2, and Caspase-3 mRNA/protein expression induced by nickel sulfate in Wistar rats. Biol Trace Elem Res 195:159–168. https://doi.org/10.1007/s12011.019.01825.0
Fedala A, Adjroud O, Abid-Essefi S, Timoumi R (2021) Protective effects of selenium and zinc against potassium dichromate–induced thyroid disruption, oxidative stress, and DNA damage in pregnant Wistar rats. Environ Sci Pollut Res 1-14. https://doi.org/10.1007/s11356.020.12268.9
Zhang T, Chen H, Liu Y (2021) Nickel Sulfate Induces Autophagy in Human Thyroid Follicular Epithelial Cells. Biol Trace Elem Res 1-12. https://doi.org/10.1007/s12011-021-02643-z
Yang J, Ma Z (2021) Research progress on the effects of nickel on hormone secretion in the endocrine axis and on target organs. Ecotoxicol Environ Saf 213:112034. https://doi.org/10.1016/j.ecoenv.2021.112034
Yoshizuka M, Mori N, Hamasaki K, et al (1991) Cadmium toxicity in the thyroid gland of pregnant rats. Exp Mol Pathol 55(1):97–104. https://doi.org/10.1016/0014-4800(91)90021-O
Prakash P, Kumar PG, Laloraya M, Javeri T, Parihar MS (1997) Superoxide anion radical production as a cadmium-mediated mechanism of toxicity in avian thyroid: An electron spin resonance study by spin trapping. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 118:89–95. https://doi.org/10.1016/S0742-8413(97)00082-0
Matović V, Buha A, Ðukić-Ćosić D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver, and kidneys. Food Chem Toxicol 78:130–40. https://doi.org/10.1016/j.fct.2015.02.011
LaBella FS, Dular R, Lemon P, Vivian S, Queen G (1973) Prolactin secretion is specifically inhibited by nickel. Nature 245:330–332. https://doi.org/10.1038/245330a0
Labella F, Dular R, Vivian S, Queen G (1973) Pituitary hormone releasing or inhibiting activity of metal ions present in hypothalamic extracts. Biochem Biophys Res Commun 52(3):786–791. https://doi.org/10.1016/0006-291X(73)91006-1
Ambali SF, Orieji C, Abubakar WO, Shittu M, Kawu MU (2011) Ameliorative effect of vitamin C on alterations in thyroid hormones concentrations induced by subchronic coadministration of chlorpyrifos and lead in Wistar rats. J Thyroid Res 2011. https://doi.org/10.4061/2011/214924
McCANN SM, Mastronardi C, Laurentiis AD, Rettori V (2005) The nitric oxide theory of aging revisited. Ann N Y Acad Sci 1057(1):64–84
Liu CM, Zheng GH, Ming QL, Chao C, Sun JM (2013) Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K-Akt pathway. J Agric Food Chem 61(5):1146-1154. https://doi.org/10.1021/jf304562b
Tsao YC, Gu PW, Liu SH, Tzeng IS, Chen JY, Luo JCJ (2017) Nickel exposure, and plasma levels of biomarkers for assessing oxidative stress in nickel electroplating workers. Biomark 22(5):455-460. https://doi.org/10.1080/1354750X.2016.1252964
Hfaïedh N, Allaqui MS, Hfaiedh M, El Feki A, Zourgui L, Croute F (2008). Protective effect of cactus (Opuntia ficus indica) cladode extract upon nickel-induced toxicity in rats. J Food ChemToxicol 46(12):3759–3763. https://doi.org/10.1016/j.fct.2008.09.059
Messaraha M, Klibetb F, Boumendjelb A, Abdennour C, Bouzerna N, Boulakoud MS, El Feki A (2012) Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp Toxicol Pathol 64(3):167–174. https://doi.org/10.1016/j.etp.2010.08.002
Boulila S, El Feka A, Oudadesse H, Kallel C, El Feki H (2014) Detoxification of rats subjected to nickel chloride by a biomaterial-based carbonated orthophosphate. Ann Pharm Fr 72(5):348–362. https://doi.org/10.1016/j.pharma.2014.03.004
Amudha K, Pari L (2011) Beneficial role of naringin, a flavanoid on nickel induced nephrotoxicity in rats. Chem Biol Interact 193(1):57–64. https://doi.org/10.1016/j.cbi.2011.05.003
Das D, Moniruzzaman M, Sarbajna A, Chakraborty BS (2017) Effect of heavy metals on tissue-specific antioxidant response in Indian major carps. Environ Sci Pollut Res 24(22):18010–18024. https://doi.org/10.1007/s11356-017-9415-5
Šulinskienė J, Bernotienė R, Baranauskienė D, Naginienė R, Stanevičienė I, Kašauskas A, Ivanov L (2019). Effect of zinc on the oxidative stress biomarkers in the brain of nickel-treated mice. Oxid Med Cell Longev 2019. https://doi.org/10.1155/2019/8549727
Adedara IA, Adegbosin AN, Abiola MA, Odunewu AA, Owoeye O, Owumi SE, Farombi EO (2020) Neurobehavioural and biochemical responses associated with exposure to binary waterborne mixtures of zinc and nickel in rats. Environ Toxicol Pharmacol 73:103294
Uner N, Oruc EO, Canli M, Sevgiler Y (2001) Effects of cypermethrin on antioxidant enzyme activities and lipid peroxidation in liver and kidney of the freshwater fish, Oreochromis niloticus and Cyprinus carpio (L). Bull Environ Contam Toxicol 67:657–664. https://doi.org/10.1007/s00128-001-0174-z
Duntas LH (2012) The evolving role of selenium in the treatment of graves' disease and ophthalmopathy. J Thyroid Res 2012. https://doi.org/10.1155/2012/736161
Sankar P, Telang AG, Manimaran A (2012) Protective effect of curcumin on cypermethrin-induced oxidative stress in Wistar rats. Experim Toxicol Pathol 64(5):487–493. https://doi.org/10.1016/j.etp.2010.11.003
Ihechiluru NB, Henry AN, Taiwo IE (2015) Heavy metal bioaccumulation and oxidative stress in Austroaeschnainermis (Dragon fly) of the Lagos Urban ecosystem. J Environ Chem Ecotoxicol 7:11–19. https://doi.org/10.5897/JECE2014.0336
Sun H, Wu W, Guo J, Xiao R, Jiang F, Zheng L, Zhang G (2016) Effects of nickel exposure on testicular function, oxidative stress, and male reproductive dysfunction in Spodoptera litura Fabricius. Chemosphere 148:178–187. https://doi.org/10.1016/j.chemosphere.2015.10.068
Hasanein P, Felegari Z (2017) Chelating effects of carnosine in ameliorating nickel-induced nephrotoxicity in rats. Can J Physiol Pharmacol 95(12):1426–1432. https://doi.org/10.1139/cjpp.2016.0647
Kubrak O, Husak VV, Rovenko BM, Poigner H, Mazepa MA, Kriews M, Abele D, Lushchak VI (2012) Tissue specificity in nickel uptake and induction of oxidative stress in kidney and spleen of goldfish Carassius auratus, exposed to waterborne nickel. J Aquat Toxicol 118:88–96. https://doi.org/10.1016/j.aquatox.2012.03.016
Mekkawy AM, Ahmed YH, El-Sakhawy MA (2020) Ameliorative effect of Nigella sativa oil and vitamin C on the thyroid gland and cerebellum of adult male albino rats exposed to Monosodium glutamate (histological, immunohistochemical, and biochemical studies). Tissue Cell 66:101391. https://doi.org/10.1016/j.tice.2020.101391
Mohamed HM, El-Twab SMA (2016) Gallic acid attenuates chromium-induced thyroid dysfunction by modulating antioxidant status and inflammatory cytokines. Enviro Toxicol Pharmacol 48:225–236. https://doi.org/10.1016/j.etap.2016.08.019
Hassanin KM, El-Kawi SHA, Hashem KS (2013) The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int J Nanomedicine 8:1713. https://doi.org/10.2147/IJN.S42736
ElBakry RH, Tawfik SM (2014) Histological study of the effect of potassium dichromate on the thyroid follicular cells of adult male albino rat and the possible protective role of ascorbic acid (vitamin C). J Microsc Ultrastruct. Environ Monit 2(3):137–150. https://doi.org/10.1016/j.jmau.2014.04.003
Andrade MN, Santos-Silva AP, Rodrigues-Pereira P, Paiva-Melo FD, de Lima Junior NC, Teixeira MP, Miranda-Alves L (2018) The environmental contaminant tributyltin leads to abnormalities in different levels of the hypothalamus-pituitary-thyroid axis in female rats. Environ Pollut 241:636–645. https://doi.org/10.1016/j.envpol.2018.06.006
Mohamed HZ, Ragab IK, Ghafeer HH (2016) A histological study on the possible protective effect of selenium against chromium-induced thyrotoxicity in adult male albino rats. Egypt J Histol 39(1):111. https://doi.org/10.1097/01.EHX.0000481747.20806.2d
Benvenga S, Micali A, Ieni A, Antonelli A, Fallahi P, Pallio G, Minutoli L (2021) The Association of Myo-Inositol and Selenium Contrasts Cadmium-Induced Thyroid C Cell Hyperplasia and Hypertrophy in Mice. Front Endocrinol 12:34. https://doi.org/10.3389/fendo.2021.608697
Yang H, Xing R, Liu S, Li P (2021) Effect of Fucoxanthin Administration on Thyroid Gland Injury Induced by Cadmium in Mice. Biol Trace Elem Res 199(5):1877–1884. https://doi.org/10.1007/s12011.020.02297.9
Hammouda F, Messaoudi I, el Hani J, Baati T, Saïd K, Kerkeni A (2008) Reversal of cadmium-induced thyroid dysfunction by selenium, zinc, or their combination in rat. Biol Trace Elem Res 126:194–203. https://doi.org/10.1007/s12011.008.8194.8
Saini S, Nair N, Saini MR (2013) Embryotoxic and teratogenic effects of nickel in Swiss albino mice during organogenetic period. BioMed Res Int 2013. https://doi.org/10.1155/2013/701439
Jemai H, Messaoudi I, Chaouch A, Kerkeni A (2007) Protective effect of zinc supplementation on blood antioxidant defense system in rats exposed to cadmium. J Trace Elem Med Biol 21(4):269–73. https://doi.org/10.1016/j.jtemb.2007.08.001
Xiao P, Jia XD, Zhong WJ, Jin XP, Nordberg G (2002) Restorative effects of zinc and selenium on cadmium-induced kidney oxidative damage in rats. Biomed Environ Sci 15(1):67–74
Brandão-Neto J, Saturnino ACRD, Leite LD et al (2006) Lack of acute zinc effect on thyrotropin-releasing hormone-stimulated thyroid-stimulating hormone secretion during oral zinc tolerance test in healthy men. Nutr Res 26(10):493–496. https://doi.org/10.1016/j.nutres.2006.08.010
Nishiyama S, Futagoishi-Suginohara Y, Matsukara M, Nakamura T, Higashi A, Shinohara M, Matsuda I (1994) Zinc supplementation alters thyroid hormone metabolism in disabled patients with zinc deficiency. J Am Coll Nutr 13(1):62–67. https://doi.org/10.1080/07315724.1994.10718373
Pekary AE, Lukaski HC, Mena I, Hershman JM (1991) Processing of TRH precursor peptides in rat brain and pituitary is zinc-dependent. Peptides 12:1025–1032. https://doi.org/10.1016/0196-9781(91)90055-T
Saygin M, Caliskan S, Karahan N, Koyu A, Gumral N, Uguz AC (2011) Testicular apoptosis and histopathological changes induced by a 2.45 GHz electromagnetic field. Toxicol Ind Health 27(5):455–463. https://doi.org/10.1177/0748233710389851
Lee SR (2018) Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longev 20:1–11. https://doi.org/10.1155/2018/9156285
Samir D, Kechrid Z, Djabar MR (2012) Combined protective effect of zinc and vitamin C on nickel-induced oxidative liver injury in rats. Annals of Biological research 3(7):3410–3418.
Adedara IA, Abiola MA, Adegbosin AN, Odunewu AA, Farombi EO (2019). Impact of binary waterborne mixtures of nickel and zinc on hypothalamic-pituitary-testicular axis in rats. Chemosphere 237:124501. https://doi.org/10.1016/j.chemosphere.2019.124501
Sidhu P, Garg ML, Dhawan DK (2006) Zinc protects rat liver histo-architecture from detrimental effects of nickel. Biometals 19(3):301–313. https://doi.org/10.1007/s10534.005.0857.8
Benvenga S, Marini HR, Micali A, Freni J, Pallio G, Irrera N, Minutoli L (2020) Protective effects of Myo-inositol and selenium on cadmium-induced thyroid toxicity in mice. Nutrients 12(5):1222. https://doi.org/10.3390/nu12051222
Atteia HH, Arafa MH, Prabahar K (2018) Selenium nanoparticles prevent lead acetate-induced hypothyroidism and oxidative damage of thyroid tissues in male rats through modulation of selenoenzymes and suppression of miR-224. Biomed Pharmacother 99:486–91. https://doi.org/10.1016/j.biopha.2018.01.083
Xing M, Jin X, Wang J, Shi Q, Cai J, Xu S (2018) The antagonistic effect of selenium on lead-induced immune dysfunction via recovery of cytokine and heat shock protein expression in chicken neutrophils. Biol Trace Elem Res 185:162–169. https://doi.org/10.1007/s12011.017.1200.2
Gao XJ, Tang B, Liang HH, Yi L, Wei ZG (2019). Selenium deficiency induced an inflammatory response by the HSP60-TLR2-MAPKs signaling pathway in the liver of carp. Fish Shellfish Immunol 87:688–694. https://doi.org/10.1016/j.fsi.2019.02.017
Schmutzler C, Mentrup B, Schomburg L, Hoang-Vu C, Herzog V, Köhrle J (2007) Selenoproteins of the thyroid gland: expression, localization and possible function of glutathione peroxidase 3. Biol Chem 388:1053–9. https://doi.org/10.1515/BC.2007.122
Drutel A, Archambeaud F, Caron P (2013) Selenium and the thyroid gland: more good news for clinicians. Clin Endocrinol 78(2):155–64. https://doi.org/10.1111/cen.12066
Cai J, Zhang Y, Yang J et al (2017) Antagonistic effects of selenium against necroptosis injury via adiponectin-necrotic pathway induced by cadmium in heart of chicken. RSC Adv 7:44438–44446. https://doi.org/10.1039/C7RA07952D
Wang N, Tan HY, Li S, Xu Y, Guo W, Feng Y (2017) Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxid Med Cell Longev 2017. https://doi.org/10.1155/2017/7478523
Duntas LH (2009) Selenium and inflammation: underlying antiinflammatory mechanisms. Horm Metab Res 41(6):443–447
Messarah M, Klibet F, Boumendjel A, Abdennour C, Bouzerna N, Boulakoud MS, El Feki A (2012) Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp Toxicol Pathol 64(3):167–174. https://doi.org/10.1016/j.etp.2010.08.002
Imed M, Fatima H, Abdelhamid K (2008) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation. Food Chem Toxicol 46(11):3522-3527. https://doi.org/10.1016/j.fct.2008.08.037
Nazari A, Fanaei H, Dehpour AR, Hassanzadeh GRE, Jafari M, Salehi M (2014) Chemical composition and hepatoprotective activity of ethanolic root extract of Taraxacum Syriacum Boiss against acetaminophen intoxication in rats. Bratisl Lek Listy 116(1):41–46
Funding
This research is supported by the General Direction of Scientific Research and Development of Technology and Ministry of Higher Education and Scientific Research, DGRSDT-MESRS (code: No. E2212600) Algeria.
Author information
Authors and Affiliations
Contributions
IS and OA designed the experiment; IS and AE performed the experiment; IS, OA, and AE analyzed the data; IS and OA wrote the manuscript; OA and AE revised the manuscript; and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All procedures were approved by the Institutional Animal Care and Use Committee of Batna University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salah, I., Adjroud, O. & Elwej, A. Protective Effects of Selenium and Zinc Against Nickel Chloride–Induced Hormonal Changes and Oxidative Damage in Thyroid of Pregnant Rats. Biol Trace Elem Res 200, 2183–2194 (2022). https://doi.org/10.1007/s12011-021-02815-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02815-x