Abstract
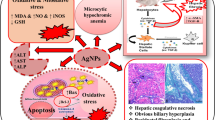
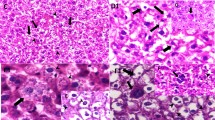
The increase in the usage of silica nanoparticles (SiNPs) in the industrial and medical fields has raised concerns about their possible adverse effects on human health. The present study aimed to investigate the potential adverse effects of SiNPs at daily doses of 25 and 100 mg/kg body weight intraperitoneally (i.p.) for 28 consecutive days on markers of liver damage in adult male rats. Results revealed that SiNPs induced a marked increase in serum markers of liver damage, including lactate dehydrogenase (LDH), alanine aminotransferase (ALAT), and aspartate aminotransferase (ASAT). SiNPs also induced an elevation of reactive oxygen species (ROS) production in liver, along with an increase in oxidative stress markers (NO, MDA, PCO, and H2O2), and a decrease in antioxidant enzyme activities (CAT, SOD, and GPx). Quantitative real-time PCR showed that SiNPs also induced upregulation of pro-apoptotic gene expression (including Bax, p53, Caspase-9/3) and downregulation of anti-apoptotic factors Bcl-2. Moreover, histopathological analysis revealed that SiNPs induced hepatocyte alterations, which was accompanied by sinusoidal dilatation, Kupffer cell hyperplasia, and the presence of inflammatory cells in the liver. Taken together, these data showed that SiNPs trigger hepatic damage through ROS-activated caspase signaling pathway, which plays a fundamental role in SiNP-induced apoptosis in the liver.






Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Abdelazeim SA, Shehata NI, Aly HF, Shams SGE (2020) Amelioration of oxidative stress-mediated apoptosis in copper oxide nanoparticles-induced liver injury in rats by potent antioxidants. Sci Rep 10:10812. https://doi.org/10.1038/s41598-020-67784-y
Aebi, H., 1984. [13] Catalase in vitro, in: Methods in Enzymology. Elsevier, pp. 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmed MA (2013) The protective effect of ginger (Zingiber officinale) against adriamycin-induced hepatotoxicity in rats: histological study. Life Sci J 10(1):1412–1422
Alkan, F.Ü., Gürsel, F.E., Ateş, A., Özyürek, M., Güçlü, K., Altun, M., 2012. Protective effects of Salvia officinalis extract against cyclophosphamide-induced genotoxicity and oxidative stress in rats 9.
Almansour M, Alarifi S, Jarrar B (2018) In vivo investigation on the chronic hepatotoxicity induced by intraperitoneal administration of 10-nm silicon dioxide nanoparticles. IJN 13:2685–2696. https://doi.org/10.2147/IJN.S162847
Aouey, B., Fares, E., Chtourou, Y., Bouchard, M., Fetoui, H., 2019. Lambda-cyhalothrin exposure alters purine nucleotide hydrolysis and nucleotidase gene expression pattern in platelets and liver of rats. Chem Biol Interact 311 108796. https://doi.org/10.1016/j.cbi.2019.108796
Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, Jacks T (2000) PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev 14:704–718
Balli, E., Yalin, S., Eroğlu, P., Bayrak, G., Çömelekoğlu, Ü., 2019. Effects of different sizes silica nanoparticle on the liver, kidney and brain in rats: biochemical and histopathological evaluation. jrp 23, 344–353. https://doi.org/10.12991/jrp.2019.142
Birk J, Meyer M, Aller I, Hansen HG, Odermatt A, Dick TP, Meyer AJ, Appenzeller-Herzog C (2013) Endoplasmic reticulum: reduced and oxidized glutathione revisited. J Cell Sci 126:1604–1617. https://doi.org/10.1242/jcs.117218
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chan W-T, Liu C-C, Chiang Chiau J-S, Tsai S-T, Liang C-K, Cheng M-L, Lee H-C, Yeung C-Y, Hou S-Y (2017) In vivo toxicologic study of larger silica nanoparticles in mice. IJN 12:3421–3432. https://doi.org/10.2147/IJN.S126823
Dilberoglu UM, Gharehpapagh B, Yaman U, Dolen M (2017) The role of additive manufacturing in the era of Industry 4.0. Procedia Manufacturing 11:545–554. https://doi.org/10.1016/j.promfg.2017.07.148
Dogra P, Adolphi NL, Wang Z, Lin Y-S, Butler KS, Durfee PN, Croissant JG, Noureddine A, Coker EN, Bearer EL, Cristini V, Brinker CJ (2018) Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat Commun 9:4551. https://doi.org/10.1038/s41467-018-06730-z
Draper, H.H., Hadley, M., 1990. [43] Malondialdehyde determination as index of lipid peroxidation, in: Methods in Enzymology. Elsevier, pp. 421–431. https://doi.org/10.1016/0076-6879(90)86135-I
Driver AS, Kodavanti PRS, Mundy WR (2000) Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 22:175–181. https://doi.org/10.1016/S0892-0362(99)00069-0
Du Z, Chen S, Cui G, Yang Y, Zhang E, Wang Q, Lavin M, Yeo A, Bo C, Zhang Y, Li C, Liu X, Yang X, Peng C, Shao H (2018) Silica nanoparticles induce cardiomyocyte apoptosis via the mitochondrial pathway in rats following intratracheal instillation. Int J Mol Med. https://doi.org/10.3892/ijmm.2018.4045
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Eom H-J, Choi J (2010) p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ Sci Technol 44:8337–8342. https://doi.org/10.1021/es1020668
Farnebo M, Bykov VJN, Wiman KG (2010) The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophys Res Commun 396:85–89. https://doi.org/10.1016/j.bbrc.2010.02.152
Fetoui H, Mahjoubi-Samet A, Jammousi K, Ellouze F, Guermazi F, Zeghal N (2006) Energy restriction in pregnant and lactating rats lowers bone mass of their progeny. Nutr Res 26:421–426. https://doi.org/10.1016/j.nutres.2006.06.016
Flohé, L., Günzler, W.A., 1984. [12] Assays of glutathione peroxidase, in: Methods in Enzymology. Elsevier, pp. 114–120. https://doi.org/10.1016/S0076-6879(84)05015-1
Gay C, Collins J, Gebicki JM (1999) Determination of iron in solutions with the ferric–xylenol orange complex. Anal Biochem 273:143–148. https://doi.org/10.1006/abio.1999.4207
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138. https://doi.org/10.1016/0003-2697(82)90118-X
Guo C, Xia Y, Niu P, Jiang L, Duan J, Yu Y, Zhou X, Li Y, Sun Z (2015) Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-κ B signaling. IJN 1463. https://doi.org/10.2147/IJN.S76114
Gupta S, Kass GEN, Szegezdi E, Joseph B (2009) The mitochondrial death pathway: a promising therapeutic target in diseases. J Cell Mol Med 13:1004–1033. https://doi.org/10.1111/j.1582-4934.2009.00697.x
Ivanov S, Zhuravsky S, Yukina G, Tomson V, Korolev D, Galagudza M (2012) In vivo toxicity of intravenously administered silica and silicon nanoparticles. Materials 5:1873–1889. https://doi.org/10.3390/ma5101873
Kanipandian N, Li D, Kannan S (2019) Induction of intrinsic apoptotic signaling pathway in A549 lung cancer cells using silver nanoparticles from Gossypium hirsutum and evaluation of in vivo toxicity. Biotechnol Rep 23:e00339. https://doi.org/10.1016/j.btre.2019.e00339
Kim J-H, Kim C-S, Ignacio RM, Kim D-H, Sajo MEJ, Maeng E, Qi X-F, Park S-E, Kim Y-R, Kim M-K, Lee K-J, Kim S-K (2014) Immunotoxicity of silicon dioxide nanoparticles with different sizes and electrostatic charge. Int J Nanomed 9(Suppl 2):183–193. https://doi.org/10.2147/IJN.S57934
Korhonen R, Lahti A, Kankaanranta H, Moilanen E (2005) Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 4:471–479. https://doi.org/10.2174/1568010054526359
Kumar, S., Eroglu, E., Stokes, J.A., Scissum-Gunn, K., Saldanha, S.N., Singh, U.P., Manne, U., Ponnazhagan, S., Mishra, M.K., 2017. Resveratrol induces mitochondria-mediated, caspase-independent apoptosis in murine prostate cancer cells. Oncotarget 8, 20895–20908. https://doi.org/10.18632/oncotarget.14947
Li J, He X, Yang Y, Li M, Xu C, Yu R (2018) Risk assessment of silica nanoparticles on liver injury in metabolic syndrome mice induced by fructose. Sci Total Environ 628–629:366–374. https://doi.org/10.1016/j.scitotenv.2018.02.047
LJVAK, K., (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2^<-ΔΔCT> method. Methods 25:402–408
Ly, J.D., Grubb, D.R., Lawen, A., 2003. The mitochondrial membrane potential (∆ψm) in apoptosis; an update 14.
Mahmoud AM, Dera HSA (2015) 18β-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: potential role of PPARγ and Nrf2 upregulation. Genes Nutr 10:1–13. https://doi.org/10.1007/s12263-015-0491-1
Mahmoud AM, Desouky EM, Hozayen WG, Bin-Jumah M, El-Nahass E-S, Soliman HA, Farghali AA (2019) Mesoporous silica nanoparticles trigger liver and kidney injury and fibrosis via altering TLR4/NF-κB, JAK2/STAT3 and Nrf2/HO-1 signaling in rats. Biomolecules 9:528. https://doi.org/10.3390/biom9100528
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
McGill, M.R., 2016. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J 15, 817–828. https://doi.org/10.17179/excli2016-800
Michael T (2008) Progress in the development of microscopical techniques for diagnostic pathology. J Histotechnol 32:9–19. https://doi.org/10.1179/his.2009.32.1.9
Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, Brassinne F, Sebaihi N, Hoet PH (2017) Toxicology of silica nanoparticles: an update. Arch Toxicol 91:2967–3010. https://doi.org/10.1007/s00204-017-1993-y
Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH (2010) The nanosilica hazard: another variable entity. Part Fibre Toxicol 7:39. https://doi.org/10.1186/1743-8977-7-39
Nemmar A, Yuvaraju P, Beegam S, Pathan J, Kazzam EE, Ali BH (2016) Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. IJN 919. https://doi.org/10.2147/IJN.S92278
Nishimori H, Kondoh M, Isoda K, Tsunoda S, Tsutsumi Y, Yagi K (2009) Silica nanoparticles as hepatotoxicants. Eur J Pharm Biopharm 72:496–501. https://doi.org/10.1016/j.ejpb.2009.02.005
Parrish AB, Freel CD, Kornbluth S (2013) Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 5:a008672–a008672. https://doi.org/10.1101/cshperspect.a008672
Pathak G, Singh S, Kumari P, Hussain Y, Raza W, Luqman S, Meena A (2020) Cirsilineol inhibits proliferation of lung squamous cell carcinoma by inducing ROS mediated apoptosis. Food Chem Toxicol 143:111550. https://doi.org/10.1016/j.fct.2020.111550
Ramaiah SK (2007) A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol 45:1551–1557. https://doi.org/10.1016/j.fct.2007.06.007
Rambanapasi C, Zeevaart J, Buntting H, Bester C, Kotze D, Hayeshi R, Grobler A (2016) Bioaccumulation and subchronic toxicity of 14 nm gold nanoparticles in rats. Molecules 21:763. https://doi.org/10.3390/molecules21060763
Reznick, A.Z., Packer, L., 1994. [38] Oxidative damage to proteins: spectrophotometric method for carbonyl assay, in: Methods in Enzymology, Oxygen Radicals in Biological Systems Part C. Academic Press, pp. 357–363. https://doi.org/10.1016/S0076-6879(94)33041-7
Roshanfekrnahzomi Z, Badpa P, Esfandiari B, Taheri S, Nouri M, Akhtari K, Shahpasand K, Falahati M (2019) Silica nanoparticles induce conformational changes of tau protein and oxidative stress and apoptosis in neuroblastoma cell line. Int J Biol Macromol 124:1312–1320. https://doi.org/10.1016/j.ijbiomac.2018.09.118
Sureshbabu A, Ryter SW, Choi ME (2015) Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol 4:208–214. https://doi.org/10.1016/j.redox.2015.01.001
Van der Zande, M.; Vandebriel, R.J.; Groot, M.J.; Kramer, E.; Herrera Rivera, Z.E.; Rasmussen, K.; Ossenkoppele, J.S.; Tromp, P.; Gremmer, E.R.; Peters, R.J.B.; et al. Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Part. Fibre Toxicol. 2014, 11, 8. https://doi.org/10.1186/1743-8977-11-8.
Wang H, Zhang F, Wen H, Shi W, Huang Q, Huang Y, Xie J, Li P, Chen J, Qin L, Zhou Y (2020) Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J Nanobiotechnol 18:8. https://doi.org/10.1186/s12951-019-0562-3
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. IJN 12:1227–1249. https://doi.org/10.2147/IJN.S121956
Williams A.B, Schumacher B (2016) p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med 6:a026070. https://doi.org/10.1101/cshperspect.a026070
Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6:1794–1807. https://doi.org/10.1021/nl061025k
Yang L, Kuang H, Zhang W, Aguilar ZP, Wei H, Xu H (2017) Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci Rep 7:3303. https://doi.org/10.1038/s41598-017-03015-1
Yousef MI, Mutar TF, Kamel MAE-N (2019) Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol Rep 6:336–346. https://doi.org/10.1016/j.toxrep.2019.04.003
Yu Y, Duan J, Li Y, Li Y, Jing L, Yang M, Wang J, Sun Z (2017) Silica nanoparticles induce liver fibrosis via TGF-β1/Smad3 pathway in ICR mice. IJN 12:6045–6057. https://doi.org/10.2147/IJN.S132304
Yu Yang, Li Yang, Wang W, Jin M, Du Z, Li Yanbo, Duan J, Yu Yongbo, Sun Z (2013) Acute toxicity of amorphous silica nanoparticles in intravenously exposed ICR mice. PLoS ONE 8:e61346. https://doi.org/10.1371/journal.pone.0061346
Zhang X, Luan J, Chen W, Fan J, Nan Y, Wang Y, Liang Y, Meng G, Ju D (2018) Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale 10:9141–9152. https://doi.org/10.1039/C8NR00554K
Zhang Y, Bai Y, Jia J, Gao N, Li Y, Zhang R, Jiang G, Yan B (2014) Perturbation of physiological systems by nanoparticles. Chem Soc Rev 43:3762–3809. https://doi.org/10.1039/C3CS60338E
Funding
This work was financially supported by the “PRD program for Research and innovation between the Tunisian Ministry of higher education and scientific research and the Kingdom of Morocco Ministry of National Education and Vocational Training “ PRD project number 20/PRD-10.”
Author information
Authors and Affiliations
Contributions
BA, KB, BG, and HF participated in the research design. The experiments were performed by BA, and KB. Data were analyzed by BA, KB, BG, and HF. AA contributed to histology analysis. BA, BG, and HF contributed to the writing of the manuscript. In addition, BA, KB, BG, HSB, MK, MB, and HF reviewed the data and the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The experimental approach and handling of animals were performed under the experiments conducted approved by the Ethical Committee Guidelines for the Care and Use of Laboratory Animals of the Faculty of Sciences of Sfax. Tunisia. It was also carried out following guidelines as set out in the “Guide for the Care and Use of Laboratory Animals” and with the authorization of the committee.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aouey, B., Boukholda, K., Gargouri, B. et al. Silica Nanoparticles Induce Hepatotoxicity by Triggering Oxidative Damage, Apoptosis, and Bax-Bcl2 Signaling Pathway. Biol Trace Elem Res 200, 1688–1698 (2022). https://doi.org/10.1007/s12011-021-02774-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02774-3