Abstract
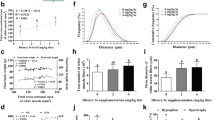
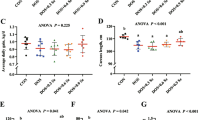
Selenium (Se) deficiency and excess can lead to protein degradation in fish. However, the underlying mechanisms remain unclear. Ubiquitin proteasome system (UPS) is the main pathway of muscle proteolysis. This study aimed to investigate the effect and molecular mechanism of dietary Se on ubiquitin-mediated muscle protein degradation in rainbow trout (Oncorhynchus mykiss). The fish were fed with the Se-deficient diet (0 mg/kg, DSe), Se-adequate diet (4 mg/kg, ASe), and Se-excessive diet (16 mg/kg, ESe), respectively. After a 10-week feeding trial, the growth performance, body composition, antioxidant enzyme activities, and UPS-related gene and protein expressions were detected. Results indicated that DSe and ESe diets significantly decreased the weight gain rate, specific growth rate, feed efficiency, and muscle crude protein content compared with ASe diet. The histological analysis showed that the mean diameter of muscle fibers was significantly decreased in DSe and ESe groups. And DSe and ESe diets significantly increased the contents of malondialdehyde and nitric oxide, but reduced the glutathione peroxidase activity. Additionally, the abundance of muscle ubiquitinated proteins and the expression levels of MuRF1 and Atrogin-1 were significantly increased in DSe and ESe groups. Compared to ASe diet, DSe and ESe diets significantly decreased the phosphorylation level of Akt Ser473 and the ratio of p-FoxO3a/FoxO3a, but significantly increased the phosphorylation level of IκBα and upregulated the expressions of TNF-α, IL-8, and NF-κB. Overall, this study indicated that dietary Se deficiency and excess accelerated the ubiquitin-mediated muscle protein degradation through regulating Akt/FoxO3a and NF-κB signaling pathways in rainbow trout.






Similar content being viewed by others
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable
References
Brigelius-Flohé R (2015) The evolving versatility of selenium in biology. Antioxid Redox Signal 23:757–760. https://doi.org/10.1089/ars.2015.6469
Khan KU, Zuberi A, Fernandes JBK, Ullah I, Sarwar H (2017) An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol Biochem 43:1689–1705. https://doi.org/10.1007/s10695-017-0402-z
Wang L, Zhang X, Wu L, Liu Q, Zhang D, Yin J (2018) Expression of selenoprotein genes in muscle is crucial for the growth of rainbow trout (Oncorhynchus mykiss) fed diets supplemented with selenium yeast. Aquaculture 492:82–90. https://doi.org/10.1016/j.aquaculture.2018.03.054
Gao X j, Tang B, Liang H h et al (2019) Selenium deficiency induced an inflammatory response by the HSP60 - TLR2 - MAPKs signalling pathway in the liver of carp. Fish Shellfish Immunol 87:688–694. https://doi.org/10.1016/j.fsi.2019.02.017
Yu H, Zhang C, Zhang X, Wang C, Li P, Liu G, Yan X, Xiong X, Zhang L, Hou J, Liu S, Zhou J, Ji H (2020) Dietary nano-selenium enhances antioxidant capacity and hypoxia tolerance of grass carp Ctenopharyngodon idella fed with high-fat diet. Aquac Nutr 26:545–557. https://doi.org/10.1111/anu.13016
Wang KY, Peng CZ, Huang JL, Huang YD, Jin MC, Geng Y (2013) The pathology of selenium deficiency in Cyprinus carpio L. J Fish Dis 36:609–615. https://doi.org/10.1111/jfd.12030
Bell JG, Pirie BJS, Adron JW, Cowey CB (1986) Some effects of selenium deficiency on glutathione peroxidase ( EC 1.11.1.9) activity and tissue pathology in rainbow trout (Salmo gairdneri). Br J Nutr 55:305–311. https://doi.org/10.1079/bjn19860038
Hilton JW, Hodson PV, Slinger SJ (1980) The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri). J Nutr 110:2527–2535. https://doi.org/10.1093/jn/110.12.2527
Naderi M, Ferrari MCO, Chivers DP, Niyogi S (2018) Maternal exposure to dietary selenium causes dopaminergic hyperfunction and cognitive impairment in zebrafish offspring. Environ Sci Technol 52:13574–13583. https://doi.org/10.1021/acs.est.8b04768
Pinto A, Juniper DT, Sanil M, Morgan L, Clark L, Sies H, Rayman MP, Steinbrenner H (2012) Supranutritional selenium induces alterations in molecular targets related to energy metabolism in skeletal muscle and visceral adipose tissue of pigs. J Inorg Biochem 114:47–54. https://doi.org/10.1016/j.jinorgbio.2012.04.011
Valente LMP, Moutou KA, Conceição LEC et al (2013) What determines growth potential and juvenile quality of farmed fish species? Rev Aquac 5. https://doi.org/10.1111/raq.12020
Ji LL, Yeo D, Kang C (2020) Muscle disuse atrophy caused by discord of intracellular signaling. Antioxid Redox Signal 33:727–744. https://doi.org/10.1089/ars.2020.8072
Johnston IA, Bower NI, Macqueen DJ (2011) Growth and the regulation of myotomal muscle mass in teleost fish. J Exp Biol 214:1617–1628. https://doi.org/10.1242/jeb.038620
Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761–771. https://doi.org/10.1016/S0092-8674(94)90462-6
Bonaldo P, Sandri M (2013) Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6:25–39. https://doi.org/10.1242/dmm.010389
Wang L, Zhang D, Li S, Wang L, Yin J, Xu Z, Zhang X (2020) Dietary selenium promotes somatic growth of rainbow trout (Oncorhynchus mykiss) by accelerating the hypertrophic growth of white muscle. Biol Trace Elem Res 199:2000–2011. https://doi.org/10.1007/s12011-020-02282-w
Jackman RW, Kandarian SC (2004) The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287:834–843. https://doi.org/10.1152/ajpcell.00579.2003
Wing SS, Lecker SH, Jagoe RT (2011) Proteolysis in illness-associated skeletal muscle atrophy: from pathways to networks. Crit Rev Clin Lab Sci 48:49–70. https://doi.org/10.3109/10408363.2011.586171
Ciechanover A (1998) The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J 17:7151–7160. https://doi.org/10.1093/emboj/17.24.7151
Bodine SC, Latres E, Baumhueter S et al (2001) Identification of ubiquitin ligases required for skeletal Muscle Atrophy. Science 294:1704–1708. https://doi.org/10.1126/science.1065874
Seiliez I, Panserat S, Skiba-Cassy S et al (2008) Feeding status regulates the polyubiquitination step of the ubiquitin-proteasome-dependent proteolysis in rainbow trout (Oncorhynchus mykiss) muscle. J Nutr 138:487–491. https://doi.org/10.1093/jn/138.3.487
Cleveland BM, Weber GM, Blemings KP, Silverstein JT (2009) Insulin-like growth factor I and genetic effects on indexes of protein degradation in response to feed deprivation in rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Comp Physiol 297:1332–1342. https://doi.org/10.1152/ajpregu.00272.2009
Sun Y, Liang X, Chen J, Tang R, Li L, Li D (2018) Change in ubiquitin proteasome system of grass carp Ctenopharyngodon idellus reared in the different stocking densities. Front Physiol 9. https://doi.org/10.3389/fphys.2018.00837
Chinkes DL (2005) Methods for measuring tissue protein breakdown rate in vivo. Curr Opin Clin Nutr Metab Care 8:534–537. https://doi.org/10.1097/01.mco.0000170754.25372.37
Kasperek GJ, Snider RD (1989) Total and myofibrillar protein degradation in isolated soleus muscles after exercise. Am J Phys:1–5. https://doi.org/10.1152/ajpendo.1989.257.1.E1
Aedo JE, Reyes AE, Avendaño-Herrera R, Molina A, Valdés JA (2015) Bacterial lipopolysaccharide induces rainbow trout myotube atrophy via Akt/FoxO1/Atrogin-1 signaling pathway. Acta Biochim Biophys Sin Shanghai 47:932–937. https://doi.org/10.1093/abbs/gmv087
Cai D, Frantz JD, Tawa NE et al (2004) IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119:285–298. https://doi.org/10.1016/j.cell.2004.09.027
Tacchi L, Bickerdike R, Secombes CJ, Martin SAM (2012) Muscle-specific RING finger (MuRF) cDNAs in Atlantic Salmon (Salmo salar) and their role as regulators of muscle protein degradation. Mar Biotechnol 14:35–45. https://doi.org/10.1007/s10126-011-9385-4
Fontagné-Dicharry S, Véron V, Larroquet L, Godin S, Wischhusen P, Aguirre P, Terrier F, Richard N, Bueno M, Bouyssière B, Antony Jesu Prabhu P, Tacon P, Kaushik SJ (2020) Effect of selenium sources in plant-based diets on antioxidant status and oxidative stress-related parameters in rainbow trout juveniles under chronic stress exposure. Aquaculture 529. https://doi.org/10.1016/j.aquaculture.2020.735684
Wischhusen P, Larroquet L, Durand T, Oger C, Galano JM, Rocher A, Vigor C, Antony Jesu Prabhu P, Véron V, Briens M, Roy J, Kaushik SJ, Fauconneau B, Fontagné-Dicharry S (2020) Oxidative stress and antioxidant response in rainbow trout fry exposed to acute hypoxia is affected by selenium nutrition of parents and during first exogenous feeding. Free Radic Biol Med 155:99–113. https://doi.org/10.1016/j.freeradbiomed.2020.05.006
Wang L, Wu L, Liu Q, Zhang DF, Yin JJ, Xu Z, Zhang XZ (2018) Improvement of flesh quality in rainbow trout (Oncorhynchus mykiss) fed supranutritional dietary selenium yeast is associated with the inhibited muscle protein degradation. Aquac Nutr 24:1351–1360. https://doi.org/10.1111/anu.12672
Fontagné-Dicharry S, Godin S, Liu H, Antony Jesu Prabhu P, Bouyssière B, Bueno M, Tacon P, Médale F, Kaushik SJ (2015) Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br J Nutr 113:1876–1887. https://doi.org/10.1017/S0007114515001300
Alami-Durante H, Bazin D, Cluzeaud M, Fontagné-Dicharry S, Kaushik S, Geurden I (2018) Effect of dietary methionine level on muscle growth mechanisms in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 483:273–285. https://doi.org/10.1016/j.aquaculture.2017.10.030
Zhang Q, Vashisht AA, O’Rourke J, Corbel SY, Moran R, Romero A, Miraglia L, Zhang J, Durrant E, Schmedt C, Sampath SC, Sampath SC (2017) The microprotein Minion controls cell fusion and muscle formation. Nat Commun 8:15564. https://doi.org/10.1038/ncomms15664
Chen Q, Li N, Zhu W, Li W, Tang S, Yu W, Gao T, Zhang J, Li J (2011) Insulin alleviates degradation of skeletal muscle protein by inhibiting the ubiquitin-proteasome system in septic rats. J Inflamm 8:1–8. https://doi.org/10.1186/1476-9255-8-13
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151:185–207. https://doi.org/10.1016/S0044-8486(96)01503-7
Liu LW, Liang XF, Li J, Fang JG, Yuan XC, Li J, Alam MS (2018) Effects of dietary selenium on growth performance and oxidative stress in juvenile grass carp Ctenopharyngodon idellus. Aquac Nutr 24:1296–1303. https://doi.org/10.1111/anu.12667
Hu JR, Huang YH, Wang GX, Wu YX, Xian JA, Wang AL, Cao JM (2016) Deficient and excess dietary selenium levels affect growth performance, blood cells apoptosis and liver HSP70 expression in juvenile yellow catfish Pelteobagrus fulvidraco. Fish Physiol Biochem 42:249–261. https://doi.org/10.1007/s10695-015-0133-y
Le KT, Fotedar R (2014) Toxic effects of excessive levels of dietary selenium in juvenile yellowtail kingfish (Seriola lalandi). Aquaculture 433:229–234. https://doi.org/10.1016/j.aquaculture.2014.06.021
Jingyuan H, Yan L, Wenjing P, Wenqiang J, Bo L, Linghong M, Qunlang Z, Hualiang L, Xianping G (2020) Dietary selenium enhances the growth and anti-oxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol 101:115–125. https://doi.org/10.1016/j.fsi.2020.03.041
KüÜkbay FZ, Yazlak H, Karaca I et al (2009) The effects of dietary organic or inorganic selenium in rainbow trout (Oncorhynchus mykiss) under crowding conditions. Aquac Nutr 15:569–576. https://doi.org/10.1111/j.1365-2095.2008.00624.x
Takahashi LS, Biller-Takahashi JD, Mansano CFM, Urbinati EC, Gimbo RY, Saita MV (2017) Long-term organic selenium supplementation overcomes the trade-off between immune and antioxidant systems in pacu (Piaractus mesopotamicus). Fish Shellfish Immunol 60:311–317. https://doi.org/10.1016/j.fsi.2016.11.060
Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: Biotic and abiotic factors. Rev Fish Biol Fish 15:75–88. https://doi.org/10.1007/s11160-005-7846-4
Branco V, Canário J, Lu J, Holmgren A, Carvalho C (2012) Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free Radic Biol Med 52:781–793. https://doi.org/10.1016/j.freeradbiomed.2011.12.002
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128. https://doi.org/10.1016/0891-5849(91)90192-6
Dawood MAO, Zommara M, Eweedah NM, Helal AI (2020) Synergistic effects of selenium nanoparticles and vitamin E on growth , immune-related gene expression, and regulation of antioxidant status of Nile tilapia (Oreochromis niloticus). Biol Trace Elem Res 195:624–635. https://doi.org/10.1007/s12011-019-01857-6
Fontagné-Dicharry S, Lataillade E, Surget A, Larroquet L, Cluzeaud M, Kaushik S (2014) Antioxidant defense system is altered by dietary oxidized lipid in first-feeding rainbow trout (Oncorhynchus mykiss). Aquaculture 424–425:220–227. https://doi.org/10.1016/j.aquaculture.2014.01.009
Ma Q (2013) Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Kang M II, Kobayashi A, Wakabayashi N et al (2004) Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci U S A 101:2046–2051. https://doi.org/10.1073/pnas.0308347100
Thomas JK, Janz DM (2016) Embryo microinjection of selenomethionine reduces hatchability and modifies oxidant responsive gene expression in zebrafish. Sci Rep 6. https://doi.org/10.1038/srep26520
Lecker SH, Solomon V, Mitch WE, Goldberg AL (1999) Muscle protein breakdown and the critical role of the ubiquitin- proteasome pathway in normal and disease states. In: J Nutr 23:98-105. https://doi.org/10.1093/jn/129.1.227s
Cleveland BM, Burr GS (2011) Proteolytic response to feeding level in rainbow trout (Oncorhynchus mykiss). Aquaculture 319:194–204. https://doi.org/10.1016/j.aquaculture.2011.06.043
Chen ZJ, Sun LJ (2009) Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell 33:275–286. https://doi.org/10.1016/j.molcel.2009.01.014
Cleveland BM, Kenney PB, Manor ML, Weber GM (2012) Effects of feeding level and sexual maturation on carcass and fillet characteristics and indices of protein degradation in rainbow trout (Oncorhynchus mykiss). Aquaculture 338–341:228–236. https://doi.org/10.1016/j.aquaculture.2012.01.032
Sanchez AMJ, Candau RB, Bernardi H (2014) FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci 71:1657–1671. https://doi.org/10.1007/s00018-013-1513-z
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403. https://doi.org/10.1016/S1097-2765(04)00211-4
Gao J, Nie W, Wang F, Guo Y (2018) Maternal selenium supplementation enhanced skeletal muscle development through increasing protein synthesis and SelW mRNA levels of their offspring. Biol Trace Elem Res 186:238–248. https://doi.org/10.1007/s12011-018-1288-z
Hall DT, Ma JF, Di Marco S, Gallouzi IE (2011) Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia. Aging (Albany NY) 3:702–715. https://doi.org/10.18632/aging.100358
Peterson JM, Bakkar N, Guttridge DC (2011) NF-κB signaling in skeletal muscle health and disease. Curr Top Dev Biol 96:85–119. https://doi.org/10.1016/B978-0-12-385940-2.00004-8
Ladner KJ, Caligiuri MA, Guttridge DC (2003) Tumor necrosis factor-regulated biphasic activation of NF-κB is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem 278:2294–2303. https://doi.org/10.1074/jbc.M207129200
Hardee JP, Counts BR, Gao S, VanderVeen BN, Fix DK, Koh HJ, Carson JA (2018) Inflammatory signalling regulates eccentric contraction-induced protein synthesis in cachectic skeletal muscle. J Cachexia Sarcopenia Muscle 9:369–383. https://doi.org/10.1002/jcsm.12271
Hirasaka K, Maeda T, Ikeda C et al (2013) Isoflavones derived from soy beans prevent MuRF1-mediated muscle atrophy in C2C12 myotubes through SIRT1 activation. J Nutr Sci Vitaminol (Tokyo) 59:317–324. https://doi.org/10.3177/jnsv.59.317
Funding
This study is supported by the National Key R&D Program of China (grant number: 2019YFD0900303) and the Fundamental Research Funds for the Central Universities (grant number: 2662019FW013).
Author information
Authors and Affiliations
Contributions
Feng Zhang: conceptualization; data curation; formal analysis; writing—original draft; writing—review and editing. Zhenlei Teng: methodology; formal analysis; writing—review and editing. Li Wang: methodology; writing—review and editing. Long Wang: formal analysis; writing—review and editing. Taotao Huang: methodology, formal analysis. Xuezhen Zhang: resources; supervision; writing—review and editing; funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Scientific Ethic Committee of Huazhong Agricultural University (no. HZAUFI-2018-017).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, F., Teng, Z., Wang, L. et al. Dietary Selenium Deficiency and Excess Accelerate Ubiquitin-Mediated Protein Degradation in the Muscle of Rainbow Trout (Oncorhynchus mykiss) via Akt/FoxO3a and NF-κB Signaling Pathways. Biol Trace Elem Res 200, 1361–1375 (2022). https://doi.org/10.1007/s12011-021-02726-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02726-x