Abstract
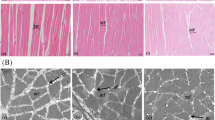
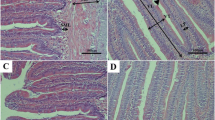
As a nutritionally essential trace element, selenium (Se) is crucial for fish growth. However, the underlying mechanisms remain unclear. Fish somatic growth relies on the white muscle growth. This study aimed to explore the effects and underlying mechanisms of Se on fish white muscle growth using a juvenile rainbow trout (Oncorhynchus mykiss) model. Fish were fed a basal diet unsupplemented or supplemented with selenium yeast at nutritional dietary Se levels (2 and 4 mg/kg Se, respectively) for 30 days. Results showed that dietary Se supplementation significantly enhanced trout somatic growth. Histological and molecular analysis of trout white muscle tissues at the vent level showed that dietary Se supplementation elevated the total cross-sectional area of white muscle, mean diameter of white muscle fibers, protein content, nuclei number, and DNA content of individual muscle fiber, and suppressed the activities of calpain system and ubiquitin-proteasome pathway. Overall, this study demonstrated that dietary Se within the nutritional range inhibits calpain- and ubiquitin-mediated protein degradation and promotes the fusion of myoblasts into the existed muscle fibers to promote the hypertrophic growth of white muscle, thereby accelerating the somatic growth of rainbow trout. Our results provide a mechanistic insight into the regulatory role of Se in fish growth.





Similar content being viewed by others
References
Khan KU, Zuberi A, Fernandes JBK, Ullah I, Sarwar H (2017) An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol Biochem 43:1689–1705. https://doi.org/10.1007/s10695-017-0402-z
Prabhu PAJ, Schrama JW, Kaushik SJ (2016) Mineral requirements of fish: a systematic review. Rev Aquacult 8:172–219. https://doi.org/10.1111/raq.12090
Lin YH, Shiau SY (2005) Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 250:356–363. https://doi.org/10.1016/j.aquaculture.2005.03.022
Bell JG, Cowey CB, Adron JW, Pirie BJS (1987) Some effects of selenium deficiency on enzyme-activities and indexes of tissue peroxidation in Atlantic salmon parr (Salmo-salar). Aquaculture 65(1):43–54. https://doi.org/10.1016/0044-8486(87)90269-9
Gao XJ, Tang B, Liang HH, Yi L, Wei ZG (2019) Selenium deficiency induced an inflammatory response by the HSP60-TLR2-MAPKs signalling pathway in the liver of carp. Fish Shellfish Immunol 87:688–694. https://doi.org/10.1016/j.fsi.2019.02.017
Zheng L, Feng L, Jiang WD, Wu P, Tang L, Kuang SY, Zeng YY, Zhou XQ, Liu Y (2018) Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 77:53–70. https://doi.org/10.1016/j.fsi.2018.03.024
Valente LMP, Moutou KA, Conceicao LEC, Engrola S, Fernandes JMO, Johnston IA (2013) What determines growth potential and juvenile quality of farmed fish species? Rev Aquacult 5:S168–S193. https://doi.org/10.1111/raq.12020
Seiliez I, Dias K, Cleveland BM (2014) Contribution of the autophagy-lysosomal and ubiquitin-proteasomal proteolytic systems to total proteolysis in rainbow trout (Oncorhynchus mykiss) myotubes. Am J Physiol Regul Integr Comp Physiol 307:R1330–R1337. https://doi.org/10.1152/ajpregu.00370.2014
Johnston IA, Bower NI, Macqueen DJ (2011) Growth and the regulation of myotomal muscle mass in teleost fish. J Exp Biol 214:1617–1628. https://doi.org/10.1242/jeb.038620
Johnston I, Strugnell G, McCracken ML, Johnstone R (1999) Muscle growth and development in normal-sex-ratio and all-female diploid and triploid Atlantic salmon. J Exp Biol 202:1991–2016
Mommsen TP (2001) Paradigms of growth in fish. Comp Biochem Phys B 129:207–219. https://doi.org/10.1016/s1096-4959(01)00312-8
Rowlerson A, Veggetti A (2001) Cellular mechanisms of post-embryonic muscle growth in aquaculture species. Fish Physiol 18:103–140. https://doi.org/10.1016/S1546-5098(01)18006-4
Weatherley A, Gill H, Lobo A (1988) Recruitment and maximal diameter of axial muscle fibres in teleosts and their relationship to somatic growth and ultimate size. J Fish Biol 33:851–859
Alami-Durante H, Medale F, Cluzeaud M, Kaushik SJ (2010) Skeletal muscle growth dynamics and expression of related genes in white and red muscles of rainbow trout fed diets with graded levels of a mixture of plant protein sources as substitutes for fishmeal. Aquaculture 303:50–58. https://doi.org/10.1016/j.aquaculture.2010.03.012
Alami-Durante H, Cluzeaud M, Duval C, Maunas P, Girod-David V, Médale F (2014) Early decrease in dietary protein:energy ratio by fat addition and ontogenetic changes in muscle growth mechanisms of rainbow trout: short- and long-term effects. Br J Nutr 112:674–687. https://doi.org/10.1017/s0007114514001391
Alami-Durante H, Bazin D, Cluzeaud M, Fontagné-Dicharry S, Kaushik S, Geurden I (2018) Effect of dietary methionine level on muscle growth mechanisms in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 483:273–285. https://doi.org/10.1016/j.aquaculture.2017.10.030
Alami-Durante H, Cluzeaud M, Bazin D, Mazurais D, Zambonino-Infante JL (2011) Dietary cholecalciferol regulates the recruitment and growth of skeletal muscle fibers and the expressions of myogenic regulatory factors and the myosin heavy chain in European sea bass larvae. J Nutr 141:2146–2151. https://doi.org/10.3945/jn.111.146118
Wang L, Zhang XZ, Wu L, Liu Q, Zhang DF, Yin JJ (2018) Expression of selenoprotein genes in muscle is crucial for the growth of rainbow trout (Oncorhynchus mykiss) fed diets supplemented with selenium yeast. Aquaculture 492:82–90. https://doi.org/10.1016/j.aquaculture.2018.03.054
Wang L, Chen CY, Liu WJ, Xia H, Li J, Zhang XZ (2017) Effects of toxic cyanobacteria and ammonia on flesh quality of blunt snout bream (Megalobrama amblycephala). J Sci Food Agric 97:1200–1206. https://doi.org/10.1002/jsfa.7850
Valente L, Rocha E, Gomes E, Silva M, Oliveira M, Monteiro R, Fauconneau B (1999) Growth dynamics of white and red muscle fibres in fast-and slow-growing strains of rainbow trout. J Fish Biol 55:675–691
Wang L, Wu L, Liu Q, Zhang DF, Yin JJ, Xu Z, Zhang X (2018) Improvement of flesh quality in rainbow trout (Oncorhynchus mykiss) fed supranutritional dietary selenium yeast is associated with the inhibited muscle protein degradation. Aquac Nutr 24:1351–1360. https://doi.org/10.1111/anu.12672
Fontagné-Dicharry S, Godin S, Liu HK, Prabhu PAJ, Bouyssière B, Bueno M, Tacon P, Médale F, Kaushik SJ (2015) Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br J Nutr 113:1876–1887. https://doi.org/10.1017/s0007114515001300
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. https://doi.org/10.1093/nar/29.9.e45
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. https://doi.org/10.1186/gb-2002-3-7-research0034
Seiliez I, Gabillard J-C, Riflade M, Sadoul B, Dias K, Avérous J, Tesseraud S, Skiba S, Panserat S (2012) Amino acids downregulate the expression of several autophagy-related genes in rainbow trout myoblasts. Autophagy 8:364–375. https://doi.org/10.4161/auto.8.3.18863
Belghit I, Skiba-Cassy S, Geurden I, Dias K, Surget A, Kaushik S, Panserat S, Seiliez I (2014) Dietary methionine availability affects the main factors involved in muscle protein turnover in rainbow trout (Oncorhynchus mykiss). Br J Nutr 112:493–503. https://doi.org/10.1017/s0007114514001226
Zhang Q, Vashisht AA, O'Rourke J, Corbel SY, Moran R, Romero A, Miraglia L, Zhang J, Durrant E, Schmedt C, Sampath SC (2017) The microprotein Minion controls cell fusion and muscle formation. Nat Commun 8. https://doi.org/10.1038/ncomms15664
Park HYL, Kim JH, Park CK (2012) Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis 3:e290. https://doi.org/10.1038/cddis.2012.26
AlamiDurante H, Fauconneau B, Rouel M, Escaffre AM, Bergot P (1997) Growth and multiplication of white skeletal muscle fibres in carp larvae in relation to somatic growth rate. J Fish Biol 50:1285–1302. https://doi.org/10.1006/jfbi.1997.0388
Valente LMP, Gomes EFS, Fauconneau B (1998) Biochemical growth characterization of fast and slow-growing rainbow trout strains: effect of cell proliferation and size. Fish Physiol Biochem 18:213–224. https://doi.org/10.1023/a:1007774929535
Hunt AO, Berkoz M, Ozkan F, Yalin S, Ercen Z, Erdogan E, Gunduz SG (2011) Effects of organic selenium on growth, muscle composition, and antioxidant system in rainbow trout. Isr J Aquacult-Bamid 63
Sivan G, Stein OE (2008) Regulation of mRNA translation during cellular division. Cell Cycle 7:741–744. https://doi.org/10.4161/cc.7.6.5596
Pestova TV, Hellen CUT (2000) The structure and function of initiation factors in eukaryotic protein synthesis. Cell Mol Life Sci 57:651–674. https://doi.org/10.1007/pl00000726
Goodman CA, Mayhew DL, Hornberger TA (2011) Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal 23:1896–1906. https://doi.org/10.1016/j.cellsig.2011.07.013
Figueiredo VC, McCarthy JJ (2019) Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology 34:30–42. https://doi.org/10.1152/physiol.00034.2018
Bennett AH, O'Donohue MF, Gundry SR, Chan AT, Widrick J, Drapers I, Chakraborty A, Zhou Y, Zon LI, Gleizes PE, Beggs AH, Gupta VA (2018) RNA helicase, DDX27 regulates skeletal muscle growth and regeneration by modulation of translational processes. PLoS Genet 14:e1007226. https://doi.org/10.1371/journal.pgen.1007226
Goll DE, Thompson VF, Li HQ, Wei W, Cong JY (2003) The calpain system. Physiol Rev 83:731–801. https://doi.org/10.1152/physrev.00029.2002
Sanchez AMJ, Candau RB, Bernardi H (2014) FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci 71:1657–1671. https://doi.org/10.1007/s00018-013-1513-z
Buckingham M, Rigby PWJ (2014) Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell 28:225–238. https://doi.org/10.1016/j.devcel.2013.12.020
Zammit PS (2017) Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol 72:19–32. https://doi.org/10.1016/j.semcdb.2017.11.011
Liu N, Nelson BR, Bezprozvannaya S, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN (2014) Requirement of MEF2A, C, and D for skeletal muscle regeneration. Proc Natl Acad Sci U S A 111:4109–4114. https://doi.org/10.1073/pnas.1401732111
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727. https://doi.org/10.1074/jbc.R800045200
Whanger PD (2009) Selenoprotein expression and function-Selenoprotein W. Biochim Biophys Acta 1790:1448–1452. https://doi.org/10.1016/j.bbagen.2009.05.010
Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Lu XG, Le XG (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141:1605–1610. https://doi.org/10.3945/jn.111.145722
Fan RF, Cao CY, Chen MH, Shi QX, Xu SW (2018) Gga-let-7f-3p promotes apoptosis in selenium deficiency-induced skeletal muscle by targeting selenoprotein K. Metallomics 10:941–952. https://doi.org/10.1039/c8mt00083b
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (grant number: 2662019FW013); the Da Bei Nong Group Promoted Project for Young Scholar of HZAU (grant number: 2017DBN018) and the Fundamental Research Funds for the Central Universities (grant number 2662015PY024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This study was approved by The Scientific Ethic Committee of Huazhong Agricultural University (no. HZAUFI-2018-017).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 731 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Zhang, D., Li, S. et al. Dietary Selenium Promotes Somatic Growth of Rainbow Trout (Oncorhynchus mykiss) by Accelerating the Hypertrophic Growth of White Muscle. Biol Trace Elem Res 199, 2000–2011 (2021). https://doi.org/10.1007/s12011-020-02282-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02282-w