Abstract
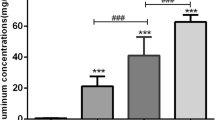
Aluminum exposure can mediate either acute toxicity or chronic toxicity. Aluminum exerts toxic effects on the cardiovascular system, but there are few studies on its related mechanisms. In this study, we investigated the molecular mechanism of aluminum-induced oxidative damage and apoptosis in rat cardiomyocytes. Thirty-two male Wistar rats were randomly divided into four groups, including the control group (GC), low-dose group of aluminum exposure (GL), medium-dose group (GM), and high-dose group (GH), with eight rats in each group. The GL, GM, and GH groups were given 5, 10, and 20 mg/(kg·d) of AlCl3 solution by intraperitoneal injection, and the GC group received intraperitoneal injection of the same volume of normal saline (2 ml/rat/day), 5 times a week for 28 days. At the end of the experiment, the levels of aluminum, malondialdehyde (MDA), plasma lactate dehydrogenase (LDH), creatine kinase (CK), creatine kinase isoenzyme (CKMB), and alpha-hydroxybutyrate dehydrogenase (HBDH) were measured. The pathological changes of myocardium were observed by H&E staining. The apoptosis of cardiomyocytes was detected by TUNEL staining, and the expression of apoptosis-related proteins was determined by western blot. The results showed that the levels of CKMB and HBDH in the GM and GH groups were significantly higher than those in the GC group (P < 0.05). The content of aluminum in the myocardium and serum of the aluminum exposure groups was significantly higher than that of the GC group (P < 0.05). The level of MDA in the GM and GH groups was significantly higher than that in the GC group (P < 0.05). The pathological results showed that vacuolated and hypertrophied cardiomyocytes were found in aluminum exposure groups, especially in the GM and GH groups. The TUNEL staining showed that the apoptosis rate of the aluminum exposure groups was considerably higher than that of the GC group (P < 0.05). Western blot showed that the expression of Bcl-2, an anti-apoptotic protein, in cardiomyocytes of aluminum exposure groups was lower than that of the GC group (P < 0.05), while the levels of Bax and caspase-3 in the cardiomyocytes of the GM and GH groups were higher than those of the GC group (P < 0.05). The experimental results showed that aluminum could accumulate in myocardial tissues and cause damage to cardiomyocytes. It could induce oxidative stress damage by increasing the content of MDA in cardiomyocytes and trigger cardiomyocyte apoptosis by activating the pro-apoptotic proteins caspase-3 and Bax and reducing the anti-apoptotic protein Bcl-2.





Similar content being viewed by others
References
Shaw CA, Tomljenovic L (2013) Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res 56(2-3):304–316. https://doi.org/10.1007/s12026-013-8403-1
Nie J (2018) Exposure to aluminum in daily life and Alzheimer’s disease. Adv Exp Med Biol 1091:99–111. https://doi.org/10.1007/978-981-13-1370-7_6
Laabbar W, Elgot A, Elhiba O, Gamrani H (2019) Curcumin prevents the midbrain dopaminergic innervations and locomotor performance deficiencies resulting from chronic aluminum exposure in rat. J Chem Neuroanat 100:101654. https://doi.org/10.1016/j.jchemneu.2019.101654
Mouro VGS, Menezes TP, Lima GDA, Domingues RR, Souza ACF, Oliveira JA, Matta SLP, Machado-Neves M (2018) How bad is aluminum exposure to reproductive parameters in rats? Biological trace element research 183(2):314–324. https://doi.org/10.1007/s12011-017-1139-3
Sun X, Liu J, Zhuang C, Yang X, Han Y, Shao B, Song M, Li Y, Zhu Y (2016) Aluminum trichloride induces bone impairment through TGF-β1/Smad signaling pathway. Toxicology 371:49–57. https://doi.org/10.1016/j.tox.2016.10.002
Balgoon MJ (2019) Assessment of the protective effect of lepidium sativum against aluminum-induced liver and kidney effects in albino rat. Biomed Res Int 2019:4516730–4516739. https://doi.org/10.1155/2019/4516730
Yuan Y, Xiao Y, Feng W, Liu Y, Yu Y, Zhou L, Qiu G, Wang H, Liu B, Liu K, Yang H, Li X, Min X, Zhang C, Xu C, Zhang X, He M, Hu FB (2017) Plasma metal concentrations and incident coronary heart disease in Chinese adults: the Dongfeng-Tongji cohort. Environmental health perspectives 125(10):107007. https://doi.org/10.1289/EHP1521
Schmidt PM, Escobar AG, Torres JG, Martinez CS, Rizzetti DA, Kunz SN, Vassallo DV, Alonso MJ, Peçanha FM, Wiggers GA (2016) Aluminum exposure for one hour decreases vascular reactivity in conductance and resistance arteries in rats. Toxicol Appl Pharmacol 313:109–118. https://doi.org/10.1016/j.taap.2016.10.023
Martinez CS, Piagette JT, Escobar AG, Martín Á, Palacios R, Peçanha FM, Vassallo DV, Exley C, Alonso MJ, Miguel M, Salaices M, Wiggers GA (2017) Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: a concerted action of NAD(P)H oxidase and COX-2. Toxicology 390:10–21. https://doi.org/10.1016/j.tox.2017.08.004
Liu Z, He C, Chen M, Yang S, Li J, Lin Y, Deng Y, Li N, Guo Y, Yu P, Li X (2018) The effects of lead and aluminum exposure on congenital heart disease and the mechanism of oxidative stress. Reprod Toxicol 81:93–98. https://doi.org/10.1016/j.reprotox.2018.07.081
Novaes RD, Mouro VGS, Gonçalves RV, Mendonça AAS, Santos EC, Fialho MCQ, Machado-Neves M (2018) Aluminum: a potentially toxic metal with dose-dependent effects on cardiac bioaccumulation, mineral distribution, DNA oxidation and microstructural remodeling. Environ Pollut 242:814–826. https://doi.org/10.1016/j.envpol.2018.07.034
Yu H, Zhang J, Ji Q, Yu K, Wang P, Song M, Cao Z, Zhang X, Li Y (2019) Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol Environ Saf 173:131–141. https://doi.org/10.1016/j.ecoenv.2019.01.095
Xu J, Lin C, Wang T, Zhang P, Liu Z, Lu C (2018) Ergosterol attenuates LPS-induced myocardial injury by modulating oxidative stress and apoptosis in rats. Cell Physiol Biochem 48(2):583–592. https://doi.org/10.1159/000491887
Sergio L, Thomé AMC, Trajano L, Mencalha AL (2018) Photobiomodulation prevents DNA fragmentation of alveolar epithelial cells and alters the mRNA levels of caspase 3 and Bcl-2 genes in acute lung injury. Photochem Photobiol Sci 17(7):975–983. https://doi.org/10.1039/c8pp00109j
Li Z, Zhao G, Qian S, Yang Z, Chen X, Chen J, Cai C, Liang X, Guo J (2012) Cerebrovascular protection of β-asarone in Alzheimer’s disease rats: a behavioral, cerebral blood flow, biochemical and genic study. J Ethnopharmacol 144(2):305–312. https://doi.org/10.1016/j.jep.2012.09.013
Cheraghi EC, Golkar A, Roshanaei K, Alani B (2017) Aluminium-induced oxidative stress, apoptosis and alterations in testicular tissue and sperm quality in Wistar rats: ameliorative effects of curcumin. Int J Fertil Steril 11(3):166–175. https://doi.org/10.22074/ijfs.2017.4859
Ghorbel I, Khemakhem M, Boudawara O, Marrekchi R, Jamoussi K, Ben Amar R, Boudawara T, Zeghal N, Grati Kamoun N (2015) Effects of dietary extra virgin olive oil and its fractions on antioxidant status and DNA damage in the heart of rats co-exposed to aluminum and acrylamide. Food Funct 6(9):3098–3108. https://doi.org/10.1039/c5fo00342c
Jeong CH, Kwon HC, Kim DH, Cheng WN, Kang S, Shin DM, Yune JH, Yoon JE, Chang YH, Sohn H, Han SG (2020) Effects of aluminum on the integrity of the intestinal epithelium: an in vitro and in vivo study. Environ Health Perspect 128(1):17013. https://doi.org/10.1289/EHP5701
Oztürk B, Ozdemir S (2015) Effects of aluminum chloride on some trace elements and erythrocyte osmotic fragility in rats. Toxicol Ind Health 31(12):1069–1077. https://doi.org/10.1177/0748233713486956
Willhite CC, Ball GL, McLellan CJ (2012) Total allowable concentrations of monomeric inorganic aluminum and hydrated aluminum silicates in drinking water. Crit Rev Toxicol 42(5):358–442. https://doi.org/10.3109/10408444.2012.674101
Galal SM, Hasan HF, Abdel-Rafei MK, El Kiki SM (2019) Synergistic effect of cranberry extract and losartan against aluminium chloride-induced hepatorenal damage associated cardiomyopathy in rats. Arch Physiol Biochem 125(4):357–366. https://doi.org/10.1080/13813455.2018.1465437
Sun X, Sun H, Yu K, Wang Z, Liu Y, Liu K, Zhu Y, Li Y (2018) Aluminum chloride causes the dysfunction of testes through inhibiting the ATPase enzyme activities and gonadotropin receptor expression in rats. Biol Trace Elem Res 183(2):296–304. https://doi.org/10.1007/s12011-017-1120-1
Martínez-Martínez S, Lozano-Vidal N, López-Maderuelo MD, Jiménez-Borreguero LJ, Armesilla ÁL, Redondo JM (2019) Cardiomyocyte calcineurin is required for the onset and progression of cardiac hypertrophy and fibrosis in adult mice. Febs j 286(1):46–65. https://doi.org/10.1111/febs.14718
Saleem N, Prasad A, Goswami SK (2018) Apocynin prevents isoproterenol-induced cardiac hypertrophy in rat. Mol Cell Biochem 445(1-2):79–88. https://doi.org/10.1007/s11010-017-3253-0
Ge J, Yu H, Li J, Lian Z, Zhang H, Fang H, Qian L (2017) Assessment of aflatoxin B1 myocardial toxicity in rats: mitochondrial damage and cellular apoptosis in cardiomyocytes induced by aflatoxin B1. J Int Med Res 45(3):1015–1023. https://doi.org/10.1177/0300060517706579
Lu Y, Feng Y, Liu D, Zhang Z, Gao K, Zhang W, Tang H (2018) Thymoquinone attenuates myocardial ischemia/reperfusion injury through activation of SIRT1 signaling. Cell Physiol Biochem 47(3):1193–1206. https://doi.org/10.1159/000490216
Kumar S, Wang G, Liu W, Ding W, Dong M, Zheng N, Ye H, Liu J (2018) Hypoxia-induced mitogenic factor promotes cardiac hypertrophy via calcium-dependent and hypoxia-inducible factor-1α mechanisms. Hypertension 72(2):331–342. https://doi.org/10.1161/HYPERTENSIONAHA.118.10845
Brasil GA, Silva-Cutini MA, Moraes FSA, Pereira TMC, Vasquez EC, Lenz D, Bissoli NS, Endringer DC, de Lima EM, Biancardi VC, Maia JF, de Andrade TU (2018) The benefits of soluble non-bacterial fraction of kefir on blood pressure and cardiac hypertrophy in hypertensive rats are mediated by an increase in baroreflex sensitivity and decrease in angiotensin-converting enzyme activity. Nutrition 51-52:66–72. https://doi.org/51-52:66-72. https://doi.org/10.1016/j.nut.2017.12.007
Haghi Aminjan H, Abtahi SR, Hazrati E, Chamanara M, Jalili M, Paknejad B (2019) Targeting of oxidative stress and inflammation through ROS/NF-kappaB pathway in phosphine-induced hepatotoxicity mitigation. Life Sci 232:116607. https://doi.org/10.1016/j.lfs.2019.116607
Ghorbel I, Maktouf S, Kallel C, Ellouze Chaabouni S, Boudawara T, Zeghal N (2015) Disruption of erythrocyte antioxidant defense system, hematological parameters, induction of pro-inflammatory cytokines and DNA damage in liver of co-exposed rats to aluminium and acrylamide. Chem Biol Interact 236:31–40. https://doi.org/10.1016/j.cbi.2015.04.020
Zhang M, Zhu J, Qin X, Zhou M, Zhang X, Gao Y, Zhang T, Xiao D, Cui W, Cai X (2019) Cardioprotection of tetrahedral DNA nanostructures in myocardial ischemia-reperfusion injury. ACS Appl Mater Interfaces 11(34):30631–30639. https://doi.org/10.1021/acsami.9b10645
Onaolapo AY, Ayeni OJ, Ogundeji MO, Ajao A, Onaolapo OJ, Owolabi AR (2019) Subchronic ketamine alters behaviour, metabolic indices and brain morphology in adolescent rats: involvement of oxidative stress, glutamate toxicity and caspase-3-mediated apoptosis. J Chem Neuroanat 96:22–33. https://doi.org/10.1016/j.jchemneu.2018.12.002
Wallach D, Kang TB, Dillon CP, Green DR (2016) Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352(6281):aaf2154. https://doi.org/10.1126/science.aaf2154
Martínez MA, Ares I, Rodríguez JL, Martínez M, Roura-Martínez D, Castellano V, Lopez-Torres B, Martínez-Larrañaga MR, Anadón A (2018) Pyrethroid insecticide lambda-cyhalothrin induces hepatic cytochrome P450 enzymes, oxidative stress and apoptosis in rats. Sci Total Environ 631-632:1371–1382. https://doi.org/10.1016/j.scitotenv.2018.03.030
Wang GX, Tu HC, Dong Y, Skanderup AJ, Wang Y, Takeda S, Ganesan YT, Han S, Liu H, Hsieh JJ, Cheng EH (2017) ΔNp63 inhibits oxidative stress-induced cell death, including ferroptosis, and cooperates with the BCL-2 family to promote clonogenic survival. Cell Rep 21(10):2926–2939. https://doi.org/10.1016/j.celrep.2017.11.030
Choudhary GS, Al-Harbi S, Almasan A (2015) Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol 1219:1–9. https://doi.org/10.1007/978-1-4939-1661-0_1
Baghaei A, Solgi R, Jafari A, Abdolghaffari AH, Golaghaei A, Asghari MH, Baeeri M, Ostad SN, Sharifzadeh M, Abdollahi M (2016) Molecular and biochemical evidence on the protection of cardiomyocytes from phosphine-induced oxidative stress, mitochondrial dysfunction and apoptosis by acetyl-L-carnitine. Environ Toxicol Pharmacol 42:30–37. https://doi.org/10.1016/j.etap.2015.12.019
Funding
This work was co-financed by the Guangxi Science Natural Foundation for Young Scientists (No. 2019JJB140036), the Middle-aged and Young Teachers’ Basic Ability Promotion Project of Guangxi (No. 2019KY0556), and the First Batch of High-level Talent Scientific Research Projects of the Affiliated Hospital of Youjiang Medical University for Nationalities in 2019 (No. R20196314).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, L., He, M., Li, X. et al. Molecular Mechanism of Aluminum-Induced Oxidative Damage and Apoptosis in Rat Cardiomyocytes. Biol Trace Elem Res 200, 308–317 (2022). https://doi.org/10.1007/s12011-021-02646-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02646-w