Abstract
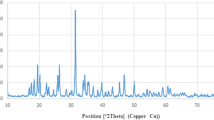
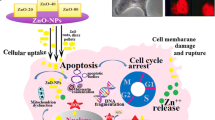
Zinc oxide nanoparticles (ZnO-NPs) are used immensely in technology and medicine, but very less is known about toxicity mechanism to human epidermal cells. The objective of this study was to evaluate possible anticancer properties of ZnO-NPs on human epidermoid carcinoma cells using MTT assay, measurement of reactive oxygen species, DNA fragmentation, and nuclear condensation. ZnO-NPs were synthesized by sol-gel method using zinc acetate dihydrate, ethylene glycol, and 2-propyl alcohol. Numerous characterization techniques such as UV-visible spectroscopy, X-ray powder diffraction, transmission electron microscopy, and dynamic light scattering spectroscopy were used to confirm synthesis, purity, optical, and surface characteristics, size, shape, and distribution of ZnO-NPs. Our finding showed that ZnO-NPs considerably decreased cell viability of human epidermoid carcinoma A431 cells with a parallel increase in nuclear condensation and DNA fragmentation in a dose dependent manner. Moreover, real time PCR expression study showed that treatment of human epidermoid carcinoma cells with ZnO-NPs trigger increased expression of tumor suppressor gene p53, bax, and caspase-3 while downregulate antiapoptotic gene bcl-2. Thus ZnO-NPs induce apoptosis in A431 cells through DNA degradation and generation of reactive oxygen species via p53, bax/bcl-2, and caspase pathways.







Similar content being viewed by others
References
Gao J, Xu B (2009) Applications of nanomaterials inside cells. Nano Today 4(1):37–51
Mann S (2008) Life as a nanoscale phenomenon. Ang Chem Int Edn 47(29):5306–5320
Wang ZL (2001) Characterization of nanophase materials. Part Part Sys Char 18(3):142–165
Whitesides GM (2003) The right size in nanobiotechnology. Nat Biotechnol 21(10):1161–1165
Khan MA, Khan MJ (2018) Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity. Art Cells Nanomed Biotechnol 46(sup1):1149–1158
Pearce ME, Melanko JB, Salem AK (2007) Multifunctional nanorods for biomedical applications. Pharm Res 24(12):2335–2352
Mamaeva V, Sahlgren C, Lindén M (2013) Mesoporous silica nanoparticles in medicine-Recent advances. Adv Drug Del Rev 65(5):689–702
Rodzinski A, Guduru R, Liang P, Hadjikhani A, Stewart T, Stimphil E, Runowicz C, Cote R, Altman N, Datar R, Khizroev S (2016) Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci Rep 6(1):20867
Alshehri R, Ilyas AM, Hasan A, Arnaout A, Ahmed F, Memic A (2016) Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J Med Chem 59(18):8149–8167
Rahong S, Yasui T, Kaji N, Baba Y (2016) Recent developments in nanowires for bio-applications from molecular to cellular levels. Lab Chip 16(7):1126–1138
Sun T, Tsang WM (2018) Nanowires for biomedical applications. In: Narayan R (ed.) Nanobiomaterials, Woodhead, pp 95-111.
Khan MJ, Svedberg A, Singh AA, Ansari MS, Karim Z (2019) Use of nanostructured polymer in the delivery of drugs for cancer therapy. In: Swain SK, Jawaid M (ed) Nanostructured Polymer Composites for Biomedical Applications, Elsevier, pp 261-276.
Zhang Y, Lim CT, Ramakrishna S, Huang Z-M (2005) Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mat Sci: Mat Med 16(10):933–946
Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI (2008) Multifunctional inorganic nanoparticles for imaging, targeting and drug delivery. ACS Nano 2(5):889–896
Szabó T, Németh J, Dékány I (2003) Zinc oxide nanoparticles incorporated in ultrathin layer silicate films and their photocatalytic properties. Colloid Surf A: Physicochem Eng Asp 230(1):23–35
Osmond-McLeod M, McCall M (2010) Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicol 4:15–41
Raghupathi KR, Koodali RT, Manna AC (2011) Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27(7):4020–4028
Fabricant RN, De Larco JE, Todaro GJ (1977) Nerve growth factor receptors on human melanoma cells in culture. Proc Nat Acad Sci USA 74(2):565–569
Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H (2014) Mechanisms of nanotoxicity: Generation of reactive oxygen species. J Food Drug Anal 22(1):64–75
Huang C-C, Aronstam RS, Chen D-R, Huang Y-W (2010) Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol in vitro 24(1):45–55
Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2(10):2121–2134
Farnebo M, Bykov VJ, Wiman KG (2010) The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophy Res Comm 396(1):85–89
Rivlin N, Brosh R, Oren M, Rotter V (2011) Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer 2(4):466–474
Roth JA, Swisher SG, Meyn RE (1999) p53 tumor suppressor gene therapy for cancer. Oncology (Williston Park, NY) 13(10 Suppl 5):148–154
Reed JC (2006) Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ 13(8):1378–1386
Czabotar PE, Lessene G, Strasser A, Adams JM (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15(1):49–63
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
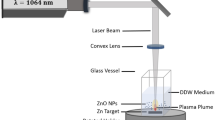
Chu S-Y, Yan T-M, Chen S-L (2000) Characteristics of sol-gel synthesis of ZnO-based powders. J Mat Sci Lett 19:349–352
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1-2):55–63
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Rad Biol Med 27(5-6):612–616
Lee EB, Cheon MG, Cui J, Lee YJ, Seo EK, Jang HH (2017) The quinone-based derivative, HMNQ induces apoptotic and autophagic cell death by modulating reactive oxygen species in cancer cells. Oncotarget 8(59):99637–99648
Kapuscinski J (1995) DAPI: a DNA-specific fluorescent probe. J Biotech Histochem 70(5):220–233
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW (2000) Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem 285(2):194–204
Rabbani G, Khan MJ, Ahmad A, Maskat MY, Khan RH (2014) Effect of copper oxide nanoparticles on the conformation and activity of β-galactosidase. Colloids and Surfaces B: Biointerfaces 123:96–105
Talam S, Karumuri SR, Gunnam N (2012) Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. ISRN Nanotechnol 2012:372505
Zak AK, Abrishami ME, Majid WHA, Yousefi R, Hosseini SM (2011) Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol-gel combustion method. Ceramics Int 37(1):393–398
Yadav A, Prasad V, Kathe AA, Raj S, Yadav D, Sundaramoorthy C, Vigneshwaran N (2006) Functional finishing in cotton fabrics using zinc oxide nanoparticles. Bull Mat Sci 29(6):641–645
Panchakarla LS, Govindaraj A, Rao CNR (2007) Formation of ZnO nanoparticles by the reaction of zinc metal with aliphatic alcohols. J Cluster Sci 18(3):660–670
Araújo Júnior EA, Nobre FX, Sousa GDS, Cavalcante LS, de Morais Chaves Santos MR, Souza FL, de Matos JME (2017) Synthesis, growth mechanism, optical properties and catalytic activity of ZnO microcrystals obtained via hydrothermal processing. RSC Adv 7(39):24263–24281
Bindu P, Thomas S (2014) Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J Theoret ApplPhys 8(4):123–134
Saraste A, Pulkki K (2000) Morphologic and biochemical markers of apoptosis. Card Res 45:528–537
Riihilä P, Nissinen L, Farshchian M, Kallajoki M, Kivisaari A, Meri S, Grénman R, Peltonen S, Peltonen J, Pihlajaniemi T, Heljasvaara R, Kähäri V-M (2017) Complement component C3 and complement factor B promote growth of cutaneous squamous cell carcinoma. Am J Pathol 187(5):1186–1197
Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, Feris K, Wingett D (2008) Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 19(29):295103–295103
Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G (2011) Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine 7(2):184–192
Bai D-P, Zhang X-F, Zhang G-L, Huang Y-F, Gurunathan S (2017) Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int J Nanomed 12:6521–6535
Ng CT, Yong LQ, Hande MP, Ong CN, Yu LE, Bay BH, Baeg GH (2017) Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int J Nanomed 12:1621–1637
Song W, Zhang J, Guo J, Zhang J, Ding F, Li L, Sun Z (2010) Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol Lett 199(3):389–397
Sherr CJ (2004) Principles of tumor suppression. Cell 116(2):235–246
Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, Hong Y (2008) DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol 233(3):404–410
Petros AM, Olejniczak ET, Fesik SW (2004) Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta 1644(2-3):83–94
Popgeorgiev N, Jabbour L, Gillet G (2018) Subcellular localization and dynamics of the Bcl-2 family of proteins. Front Cell Dev Biol 6:13–13
Guo D, Bi H, Liu B, Wu Q, Wang D, Cui Y (2013) Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol In Vitro 27(2):731–738
Sharma V, Anderson D, Dhawan A (2012) Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 17(8):852–870
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122(Pt 4):437–441
Puthalakath H, Strasser A (2002) Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 9(5):505–512
Johnstone RW, Frew AJ, Smyth MJ (2008) The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer 8(10):782–798
Krammer PH, Arnold R, Lavrik IN (2007) Life and death in peripheral T cells. Nat Rev Immunol 7(7):532–542
Roderick HL, Cook SJ (2008) Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer 8(5):361–375
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach. Nat Rrev Drug Discov 8(7):579–591
Funding
This work was supported by Deanship of Scientific Research, King Abdulaziz University, Saudi Arabia (Grant Number: DF-708-130-1441). AA thanks to DSR, King Abdulaziz University, Saudi Arabia for the financial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, M.J., Ahmad, A., Khan, M.A. et al. Zinc Oxide Nanoparticle Induces Apoptosis in Human Epidermoid Carcinoma Cells Through Reactive Oxygen Species and DNA Degradation. Biol Trace Elem Res 199, 2172–2181 (2021). https://doi.org/10.1007/s12011-020-02323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02323-4