Abstract
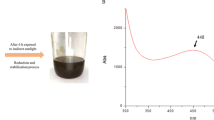
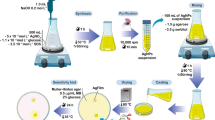
Candida species are the most common causative agents responsible for the majority of morbidity as well as mortality rates due to invasive fungal infections worldwide. In this study, a green approach was developed to control the pathogenic Candida spp. isolated from clinical samples, and prior data collections, ethics approval was obtained. Sixty candida isolates were obtained from the different device-associated infections and identified as Candida albicans, Candida tropicalis, Candida krusei, Candida parapsilosis, and Candida glabrata with prevalence rates 41.6, 38.3, 8.3, 6.6, and 5%, respectively. On the other hand, silver nanoparticles (Ag-NPs) were extra-cellular synthesized by biomass filtrate of previously identified Penicillium chrysogenum strain F9. The physico-chemical characterizations of biosynthesized Ag-NPs were assessed by using UV-Vis spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD) patterns, transmission electron microscope (TEM), dynamic light scattering (DIS), and zeta potential (ζ) analysis. Data revealed successful synthesis of crystallographic spherical Ag-NPs with average size 18 to 60 nm at maximum absorption peak 415 nm. FT-IR analysis confirmed the presence of functional groups related to reduction, capping, and stabilizing Ag-NPs. The DLS analysis showed that NPs were homogenous and stable with poly-dispersity index (PDI) and ζ value 0.008 and − 21 mV, respectively. Susceptibility pattern analysis revealed that sixty Candida isolates (100%) were susceptible to Ag-NPs as compared to 25 isolates (41.6%), and 30 isolates (50%) were susceptible to fluconazole and amphotericin B, respectively. Interestingly, 30 Candida isolates (50%) were resistant to amphotericin B, which are more than those recorded for fluconazole (17 isolates with percent 28.3%), while 18 candida isolates (30%) were susceptible dose-dependent to fluconazole. The recorded minimum inhibitory concentration 50/90 (MIC50/90) was 62.5/125, 16/64, and 1/4 for Ag-NPs, fluconazole, and amphotericin B, respectively. However, green synthesized Ag-NPs can be used to overcome the resistance pattern of Candida spp., and recommended as an anti-candida agent.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Tóth R, Nosek J, Mora-Montes HM, Gabaldon T, Bliss JM, Nosanchuk JD, Turner SA, Butler G, Vágvölgyi C, Gácser A (2019) Candida parapsilosis: from genes to the bedside. Clin Microbiol Rev 32(2):e00111–e00118
Enoch DA, Yang H, Aliyu SH, Micallef C (2017) The changing epidemiology of invasive fungal infections. In: Human fungal pathogen identification. Springer, pp 17-65
Ghaddar N, Anastasiadis E, Halimeh R, Ghaddar A, Dhar R, AlFouzan W, Yusef H, El Chaar M (2020) Prevalence and antifungal susceptibility of Candida albicans causing vaginal discharge among pregnant women in Lebanon. BMC Infect Dis 20(1):1–9
Organization WH (2014) Antimicrobial resistance: global report on surveillance. World Health Organization,
Garcia-Perez JE, Mathé L, Humblet-Baron S, Braem A, Lagrou K, Van Dijck P, Liston A (2018) A framework for understanding the evasion of host immunity by Candida Biofilms. Front Immunol 9:538
Li W, Hu Y-a, F-q L, L-n S, H-f S, Huang M, Wang Y, D-d H, Liao H, Ma C-f (2015) Distribution of yeast isolates from invasive infections and their in vitro susceptibility to antifungal agents: evidence from 299 cases in a 3-year (2010 to 2012) surveillance study. Mycopathologia 179(5-6):397–405
Lee W-J, Hsu J-F, Lai M-Y, Chiang M-C, Lin H-C, Huang H-R, Wu I-H, Chu S-M, Fu R-H, Tsai M-H (2018) Factors and outcomes associated with candidemia caused by non-albicans Candida spp versus Candida albicans in children. Am J Infect Control 46(12):1387–1393
Martins N, Barros L, Henriques M, Silva S, Ferreira IC (2015) Activity of phenolic compounds from plant origin against Candida species. Ind Crop Prod 74:648–670
Salem SS, Fouda A (2020) Green synthesis of metallic nanoparticles and their prosective biotechnological applications: an overview. Biol Trace Elem Res.https://doi.org/10.1007/s12011-020-02138-3
Al-Nuairi AG, Mosa KA, Mohammad MG, El-Keblawy A, Soliman S, Alawadhi H (2019) Biosynthesis, characterization, and evaluation of the cytotoxic effects of biologically synthesized silver nanoparticles from cyperus conglomeratus root extracts on breast cancer cell line MCF-7. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01791-7
Taha ZK, Hawar SN, Sulaiman GM (2019) Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotechnol Lett 41(8–9):899–914
Hassan SE-D, Fouda A, Radwan AA, Salem SS, Barghoth MG, Awad MA, Abdo AM, El-Gamal MS (2019) Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. JBIC J Biol Inorganic Chem 24(3):377–393. https://doi.org/10.1007/s00775-019-01654-5
Fouda A, Saad E, Salem SS, Shaheen TI (2018) In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized zinc oxide nanoparticles for medical textile applications. Microb Pathog 125:252–261
Sharaf OM, Al-Gamal MS, Ibrahim GA, Dabiza NM, Salem SS, El-ssayad MF, Youssef AM (2019) Evaluation and characterization of some protective culture metabolites in free and nano-chitosan-loaded forms against common contaminants of Egyptian cheese. Carbohydr Polym 223:115094
Shaheen TI, Salem SS, Zaghloul S (2019) A new facile strategy for multifunctional textiles development through in situ deposition of SiO2/TiO2 nanosols hybrid. Ind Eng Chem Res 58(44):20203–20212
Fouda A, Abdel-Maksoud G, Abdel-Rahman MA, Eid AM, Barghoth MG, El-Sadany MA-H (2019) Monitoring the effect of biosynthesized nanoparticles against biodeterioration of cellulose-based materials by Aspergillus niger. Cellulose 26(11):6583–6597
Calderón-Jiménez B, Johnson ME, Montoro Bustos AR, Murphy KE, Winchester MR, Vega Baudrit JR (2017) Silver nanoparticles: technological advances, societal impacts, and metrological challenges. Frontiers in chemistry 5:6
Elfeky AS, Salem SS, Elzaref AS, Owda ME, Eladawy HA, Saeed AM, Awad MA, Abou-Zeid RE, Fouda A (2020) Multifunctional cellulose nanocrystal/metal oxide hybrid, photo-degradation, antibacterial and larvicidal activities. Carbohydr Polym 230:115711
Rajeswaran S, Thirugnanasambandan SS, Dewangan NK, Moorthy RK, Kandasamy S, Vilwanathan R (2019) Multifarious pharmacological applications of green routed eco-friendly iron nanoparticles synthesized by Streptomyces Sp. (SRT12). Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01777-5
Rad M, Taran M, Alavi M (2018) Effect of incubation time, CuSO4 and glucose concentrations on biosynthesis of copper oxide (CuO) nanoparticles with rectangular shape and antibacterial activity: Taguchi method approach. Nano Biomed Eng 10(1):25–33
Luo K, Jung S, Park K-H, Kim Y-R (2018) Microbial biosynthesis of silver nanoparticles in different culture media. J Agric Food Chem 66(4):957–962
Saha N, Gupta SD (2017) Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. J Hazard Mater 330:18–28
Hassan SELD, Salem SS, Fouda A, Awad MA, El-Gamal MS, Abdo AM (2018) New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J Radiat Res Appl Sci 11(3):262–270. https://doi.org/10.1016/j.jrras.2018.05.003
Kahzad N, Salehzadeh A (2020) Green synthesis of CuFe2O4@ Ag nanocomposite using the chlorella vulgaris and evaluation of its effect on the expression of norA efflux pump gene among Staphylococcus aureus strains. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02055-5
Fouda A, Hassan SE-D, Abdo AM, El-Gamal MS (2019) Antimicrobial, antioxidant and larvicidal activities of spherical silver nanoparticles synthesized by endophytic Streptomyces spp. Biol Trace Elem Res 195:707–724. https://doi.org/10.1007/s12011-019-01883-4
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine 12:1227–1249
Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S (2014) Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 4(3):316–335
Parashar UK, Saxena PS, Srivastava A (2009) Bioinspired synthesis of silver nanoparticles. Digest Journal of Nanomaterials & Biostructures (DJNB) 4 (1)
Mohmed AA, Saad E, Fouda A, Elgamal MS, Salem SS (2017) Extracellular biosynthesis of silver nanoparticles using Aspergillus sp. and evaluation of their antibacterial and cytotoxicity. J Appl Life Sci Int. https://doi.org/10.9734/JALSI/2017/33491
Cyril N, George JB, Joseph L, Raghavamenon A, VP S (2019) Assessment of antioxidant, antibacterial and anti-proliferative (lung cancer cell line A549) activities of green synthesized silver nanoparticles from Derris trifoliata. Toxicol Res 8 (2):297–308
Mohamed NA, Pathmanathan SG, Hussin H, Zaini AB (2018) Distribution and antifungal susceptibility pattern of Candida species at a tertiary hospital in Malaysia. J Infect Dev Countries 12(02):102–108
Baradkar V, Mathur M, Kumar S (2010) Hichrom Candida agar for identification of Candida species. Indian J Pathol Microbiol 53(1):93–95
Fouda A, Abdel-Maksoud G, Abdel-Rahman MA, Salem SS, Hassan SE-D, El-Sadany MA-H (2019) Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. Int Biodeterior Biodegradation 142:160–169. https://doi.org/10.1016/j.ibiod.2019.05.012
Elamawi RM, Al-Harbi RE, Hendi AA (2018) Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt J Biological Pest Control 28(1):28
Rolim WR, Pelegrino MT, de Araújo LB, Ferraz LS, Costa FN, Bernardes JS, Rodigues T, Brocchi M, Seabra AB (2019) Green tea extract mediated biogenic synthesis of silver nanoparticles: characterization, cytotoxicity evaluation and antibacterial activity. Appl Surf Sci 463:66–74
Lass-Flörl C, Arendrup M, Rodriguez-Tudela J-L, Cuenca-Estrella M, Donnelly P, Hope W, Testing ECoAS (2011) EUCAST technical note on amphotericin B. Clin Microbiol Infect 17(12):E27–E29
Vázquez-González D, Perusquía-Ortiz AM, Hundeiker M, Bonifaz A (2013) Opportunistic yeast infections: candidiasis, cryptococcosis, trichosporonosis and geotrichosis. JDDG: J Deutschen Dermatologischen Gesellschaft 11(5):381–394
Liu X, Fan S, Bai F, Li J, Liao Q (2009) Antifungal susceptibility and genotypes of Candida albicans strains from patients with vulvovaginal candidiasis. Mycoses 52(1):24–28
Sobel JD (2007) Vulvovaginal candidosis. Lancet 369(9577):1961–1971
Matare T, Nziramasanga P, Gwanzura L (2017) Robertson V (2017) Experimental germ tube induction in Candida albicans: an evaluation of the effect of sodium bicarbonate on morphogenesis and comparison with pooled human serum. Biomed Res Int 2017:1–5
Gahlot G, Soni S, Gandhi P, Patel S, Katara R, Vegad M, Savalia A (2013) Effectiveness of CHROM agar Candida, a differential isolation medium for rapid identification of clinically important Candida species. Int J Microbiol Res 5(6):494
Silva F, Ferreira S, Duarte A, Mendonca DI, Domingues FC (2011) Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 19(1):42–47
Masri SN, Noor SM, Nor LAM, Osman M, Rahman MM (2015) Candida isolates from pregnant women and their antifungal susceptibility in a Malaysian tertiary-care hospital. Pakistan J Med Sci 31(3):658
Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C (2011) Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One 6(9)
Pfaller M, Diekema D, Gibbs D, Newell V, Ellis D, Tullio V, Rodloff A, Fu W, Ling T (2010) Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol 48(4):1366–1377
Morace G, Borghi E, Iatta R, Amato G, Andreoni S, Brigante G, Farina C, Cascio GL, Lombardi G, Manso E (2011) Antifungal susceptibility of invasive yeast isolates in Italy: the GISIA3 study in critically ill patients. BMC Infect Dis 11(1):130
Ghorbani HR, Alizadeh V, Mehr FP, Jafarpourgolroudbary H, Erfan K, Yeganeh SS (2018) Preparation of polyurethane/CuO coating film and the study of antifungal activity. Prog Org Coat 123:322–325
Majeed S, Danish M, Zahrudin AHB, Dash GK (2018) Biosynthesis and characterization of silver nanoparticles from fungal species and its antibacterial and anticancer effect. Karbala Int J Modern Sci 4(1):86–92
Alsharif SM, Salem SS, Abdel-Rahman MA, Fouda A, Eid AM, Hassan SE, Awad MA, Mohamed AA, (2020) Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 6 (5):e03943
Moshfegh A, Jalali A, Salehzadeh A, Jozani AS (2019) Biological synthesis of silver nanoparticles by cell-free extract of Polysiphonia algae and their anticancer activity against breast cancer MCF-7 cell lines. Micro & Nano Letters 14(5):581–584
Khanra K, Panja S, Choudhuri I, Chakraborty A, Bhattacharyya N (2015) Evaluation of antibacterial activity and cytotoxicity of green synthesized silver nanoparticles using Scoparia dulcis. Nano Biomed Eng 7(3):128–133
Dong Z-Y, Rao N, Prabhu M, Xiao M, Wang H-F, Hozzein WN, Chen W, Li W-J (2017) Antibacterial activity of silver nanoparticles against Staphylococcus warneri synthesized using endophytic bacteria by photo-irradiation. Front Microbiol 8:1090
Salem SS, Fouda MMG, Fouda A, Awad MA, Al-Olayan EM, Allam AA, Shaheen TI (2020) Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using penicillium corylophilum. J Clust Sci. https://doi.org/10.1007/s10876-020-01794-8
Weidner T, Breen NF, Drobny GP, Castner DG (2009) Amide or amine: determining the origin of the 3300 cm-1 NH mode in protein SFG spectra using 15 N isotope labels. J Phys Chem B 113(47):15423–15426
Melo-Silveira RF, Fidelis GP, Costa MSSP, Telles CBS, Dantas-Santos N, Elias SO, Ribeiro VB, Barth AL, Macedo AJ, Leite EL (2012) In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int J Mol Sci 13(1):409–426
Pourfarzad A, Najafi MBH, Khodaparast MHH, Khayyat MH (2015) Serish inulin and wheat biopolymers interactions in model systems as a basis for understanding the impact of inulin on bread properties: a FTIR investigation. J Food Sci Technol 52(12):7964–7973
de Oliveira Silva BS, Seabra AB (2016) Characterization of iron nanoparticles produced with green tea extract: a promising material for nitric oxide delivery. Biointerface Res Appl Chem 6(3)
Othman AM, Elsayed MA, Elshafei AM, Hassan MM (2016) Nano-silver biosynthesis using culture supernatant of Penicillium politans NRC510: optimization, characterization and its antimicrobial activity. Int J ChemTech Res 9:433–444
Othman AM, Elsayed MA, Al-Balakocy NG, Hassan MM, Elshafei AM (2019) Biosynthesis and characterization of silver nanoparticles induced by fungal proteins and its application in different biological activities. J Genet Eng Biotechn 17(1):8
Brozek-Pluska B, Kopec M, Niedzwiecka I, Morawiec-Sztandera A (2015) Label-free determination of lipid composition and secondary protein structure of human salivary noncancerous and cancerous tissues by Raman microspectroscopy. Analyst 140(7):2107–2113
Fathy RM, Salem MSE-d, Mahfouz AY (2019) Biogenic synthesis of silver nanoparticles using Gliocladium deliquescens and their application as household sponge disinfectant. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01958-2
Ebrahimzadeh Z, Salehzadeh A, Naeemi A, Jalali A (2020) Silver nanoparticles biosynthesized by Anabaena flos-aquae enhance the apoptosis in breast cancer cell line. Bull Mater Sci 43(1):1–7
Philip D (2011) Mangifera indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 78(1):327–331
Sudha A, Jeyakanthan J, Srinivasan P (2017) Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resource-Efficient Technologies 3(4):506–515
Mohamed A A, Fouda A, Elgamal SM, El-Din Hassan S, Shaheen IT, SalemSS (2017) Enhancing of cotton fabric antibacterial properties by silver nanoparticles synthesized by new Egyptian strain Fusarium keratoplasticum A1-3. Egyptian Journal of chemistry 60. Conference issue. The 8th International Conference of The Textile Research Division (ICTRD 2017), National Research Centre, Cairo, pp 63–71
Fouda A, Mohamed A, Elgamal MS, EL-Din Hassan S, Salem Salem S, Shaheen TI (2017) Facile approach towards medical textiles via myco-synthesis of silver nanoparticles. Der Pharma Chemica 9
Rai M, Ingle AP, Gade A, Duran N (2014) Synthesis of silver nanoparticles by Phoma gardeniae and in vitro evaluation of their efficacy against human disease-causing bacteria and fungi. IET nanobiotechnology 9(2):71–75
Mohamed AA, Fouda A, Abdel-Rahman MA, Hassan SE-D, El-Gamal MS, Salem SS, Shaheen TI (2019) Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatalysis and Agric Biotechnol 19:101103
Kim S-H, Lee H-S, Ryu D-S, Choi S-J, Lee D-S (2011) Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J Microbiol Biotechnol 39(1):77–85
Tomaszewska E, Soliwoda K, Kadziola K, Tkacz-Szczesna B, Celichowski G, Cichomski M, Szmaja W, Grobelny J (2013) Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J Nanomater 2013:1–10
Aziz N, Fatma T, Varma A, Prasad R (2014) Biogenic synthesis of silver nanoparticles using Scenedesmus abundans and evaluation of their antibacterial activity. J Nanomater 2014:1–6
Vu NK, Zille A, Oliveira FR, Carneiro N, Souto AP (2013) Effect of particle size on silver nanoparticle deposition onto dielectric barrier discharge (DBD) plasma functionalized polyamide fabric. Plasma Process Polym 10(3):285–296
Khan SS, Mukherjee A, Chandrasekaran N (2011) Impact of exopolysaccharides on the stability of silver nanoparticles in water. Water Res 45(16):5184–5190
Singh T, Jyoti K, Patnaik A, Singh A, Chauhan R, Chandel S (2017) Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J Genet Eng Biotechnol 15(1):31–39
Shaheen TI, Fouda A (2018) Green approach for one-pot synthesis of silver nanorod using cellulose nanocrystal and their cytotoxicity and antibacterial assessment. Int J Biol Macromol 106:784–792
Sanjenbam P, Gopal J, Kannabiran K (2014) Anticandidal activity of silver nanoparticles synthesized using Streptomyces sp. VITPK1. J Mycol Méd 24(3):211–219
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7(1):17–28
Vazquez-Muñoz R, Avalos-Borja M, Castro-Longoria E (2014) Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS One 9(10):e108876
Taj-Aldeen S, Kolecka A, Boesten R, Alolaqi A, Almaslamani M, Chandra P, Meis J, Boekhout T (2014) Epidemiology of candidemia in Qatar, the Middle East: performance of MALDI-TOF MS for the identification of Candida species, species distribution, outcome, and susceptibility pattern. Infection 42(2):393–404
Fothergill AW, Sutton DA, McCarthy DI, Wiederhold NP (2014) Impact of new antifungal breakpoints on antifungal resistance in Candida species. J Clin Microbiol 52(3):994–997
Dagi HT, Findik D, Senkeles C, Arslan U (2016) Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Ann Clin Microbiol Antimicrob 15(1):36
Zhang L, Yang H-F, Liu Y-Y, Xu X-H, Ye Y, Li J-B (2013) Reduced susceptibility of Candida albicans clinical isolates to azoles and detection of mutations in the ERG11 gene. Diagn Microbiol Infect Dis 77(4):327–329
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Ethical Committee (FMASU REC/ 464/2017) of the Faculty of Medicine, Ain Shams University, Cairo, Egypt.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soliman, A.M., Abdel-Latif, W., Shehata, I.H. et al. Green Approach to Overcome the Resistance Pattern of Candida spp. Using Biosynthesized Silver Nanoparticles Fabricated by Penicillium chrysogenum F9. Biol Trace Elem Res 199, 800–811 (2021). https://doi.org/10.1007/s12011-020-02188-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02188-7