Abstract
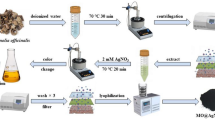
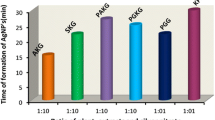
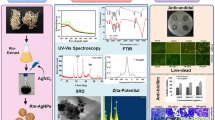
Metallic nanoparticles with antimicrobial properties have obtained the status of a new generation of antifungal drugs. This study described a method for the synthesis of biogenic silver nanoparticles (Bio-AgNPs) using cell-free supernatant containing enterocin from Enterococcus durans and assessed the antifungal potential against fluconazole-resistant Candida tropicalis. Bio-AgNPs was confirmed visually by the appearance of a reddish-brown color formation. A sharp absorbance peak at 448 nm was found using a UV-visible spectrophotometer, confirming the presence of Bio-AgNPs. Fourier transform infrared spectroscopy, differential light scattering, energy dispersive spectroscopy, and transmission electronic microscopy analyses were employed to characterize the Bio-AgNP. Reduction of Ag+ ions in solution with CFS from E. durans produced spherical Bio-AgNPs with an average size of 169.11 nm and zeta potential of − 25.3 mV. The anti-Candida activity of Bio-AgNPs and its synergism with fluconazole were determined using agar well diffusion and broth microdilution method. Bio-AgNPs at a concentration of 32.17 μg/mL promoted zones of inhibition that ranged from 18.5 ± 0.7 to 23 ± 1.7 mm among C. tropicalis strains. The minimum inhibitory concentration of Bio-AgNPs that inhibit 80% of growth ranged from 0.251 to 2.01 μg/mL. Bio-AgNPs and fluconazole showed synergistic interaction in 4 out of 6 strains (FICI range 0.23–0.31). These results demonstrate a sustainable and cost-effective method for synthesis of Bio-AgNPs with activity against C. tropicalis. Due to significant activity against fluconazole-resistant strains of C. tropicalis, Bio-AgNPs alone and in combination with fluconazole may represent an alternative in the control of infections associated with this pathogen.





Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Kullberg, B. J., & Arendrup, M. C. (2015). Invasive candidiasis. New England Journal of Medicine, 373(15), 1445–1456. https://doi.org/10.1056/NEJMra1315399
Arendrup, M. C., & Patterson, T. F. (2017). Multidrug-resistant Candida: Epidemiology, molecular mechanisms, and treatment. The Journal of Infectious Diseases, 216, 445–451. https://doi.org/10.1093/infdis/jix131
Zuza-Alves, D. L., Silva-Rocha, W. P., & Chaves, G. M. (2017). An update on Candida tropicalis based on basic and clinical approaches. Frontiers in Microbiology, 8, 1927. https://doi.org/10.3389/fmicb.2017.01927
Whaley, S. G., Berbow, E. L., Rybak, J. M., Nishimoto, A. T., Barker, K. S., & Rogers, P. D. (2017). Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Frontiers in Microbiology, 7, 2173. https://doi.org/10.3389/fmicb.2016.02173
Souza, C. M., Perini, H. F., Caloni, C. C., Furlaneto-Maia, L., & Furlaneto, M. C. (2020). Adhesion of Candida tropicalis to polystyrene and epithelial cell lines: Insights of correlation of the extent of adherent yeast cells among distinct surfaces. Journal de Mycologie Medicale, 30, 101043. https://doi.org/10.1016/j.mycmed.2020.101043
Turner, S. A., & Butler, G. (2014). The Candida pathogenic species complex. Cold Spring Harbor Perspective in Medicine, 4(9), a019778. https://doi.org/10.1101/cshperspect.a019778
de Oliveira, J. S., Pereira, V. S., de Souza Collares Maia Castelo-Branco, D., de Aguiar Cordeiro, R., Sidrim, J. J. C., Brilhante, R. S. N., & Rocha, M. F. G. (2020). The yeast, the antifungal, and the wardrobe: A journey into antifungal resistance mechanisms of Candida tropicalis. Canadian Science Publishing, 66(6), 377–388. https://doi.org/10.1139/cjm-2019-0531
WHO, (2022). WHO fungal priority pathogens list to guide research. Development and Public Health Action. World Health Organization, Geneva. License: CC BY-NC-SA3.0 IGO ISBN: 978-92-4-006024-1.
Ahamed, M., Posgai, R., Gorey, T. J., Nielsen, M., Hussain, S. M., & Rowe, J. J. (2010). Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicology and Applied Pharmacology, 242(3), 263–269. https://doi.org/10.1016/j.taap.2009.10.016
Jia, D., & Sun, W. (2021). Silver nanoparticles offer a synergistic effect with fluconazole against fluconazole-resistant Candida albicans by abrogating drug efflux pumps and increasing endogenous ROS. Infection, Genetics and Evolution, 93, 1567–1348. https://doi.org/10.1016/j.meegid.2021.104937
Feng, W., Yang, J., Xi, Z., Ji, Y., Zhu, X., Yang, L., & Ma, Y. (2019). Regulatory role of ERG3 and Efg1 in azoles-resistant strains of Candida albicans isolated from patients diagnosed with vulvovaginal candidiasis. Indian Journal of Microbiology, 59(4), 514–524. https://doi.org/10.1007/s12088-019-00833-x
Fonseca, M. S., Rodrigues, D. M., Sokolonski, A. R., Stanisic, D., Tomé, L. M., Góes-Neto, A., Azevedo, V., Meyer, R., Araújo, D. B., Tasic, L., & Portela, R. D. (2022). Activity of Fusarium oxysporum-based silver nanoparticles on Candida spp. oral isolates. Nanomaterials, 12(3), 501. https://doi.org/10.3390/nano12030501
Radhakrishnan, V. S., Mudiam, M. K. R., Kumar, M., Dwivedi, S. P., Singh, S. P., & Prasad, T. (2018). Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). International Journal of Nanomedicine, 3(13), 2647–2663. https://doi.org/10.2147/IJN.S150648
Dey, N., Kamatchi, C., Vickram, A. S., Anbarasu, K., Thanigaivel, S., Palanivelu, J., Pugazhendhi, A., & Ponnusamy, V. K. (2022). Role of nanomaterials in deactivating multiple drug resistance efflux pumps - A review. Environmental Research, 204, 111968. https://doi.org/10.1016/j.envres.2021.111968
Jardón-Romero, E. A., Lara-Carrillo, E., González-Pedroza, M. G., Sánchez-Mendieta, V., Salmerón-Valdés, E. N., Toral-Rizo, V. H., Olea-Mejía, O. F., López-González, S., & Morales-Luckie, R. A. (2022). Antimicrobial activity of biogenic silver nanoparticles from Syzygium aromaticum against the five most common microorganisms in the oral cavity. Antibiotics, 11(7), 834. https://doi.org/10.3390/antibiotics11070834
Saqib, S., Faryad, S., Afridi, M. I., Arshad, B., Younas, M., Naeem, M., Zaman, W., Ullah, F., Nisar, M., Ali, S., Elgorban, A. M., Syed, A., Elansary, H. O., & El-Abedin, T. K. Z. (2022). Bimetallic assembled silver nanoparticles impregnated in Aspergillus fumigatus extract damage the bacterial membrane surface and release cellular contents. Coatings, 12, 1505. https://doi.org/10.3390/coatings12101505
Sharif, M. S., Hameed, H., Waheed, A., Tariq, M., Afreen, A., Kamal, A., Mahmoud, E. A., Elansary, H. O., Saqib, S., & Zaman, W. (2023). Biofabrication of Fe3O4 nanoparticles from Spirogyra hyalina and Ajuga bracteosa and their antibacterial applications. Molecules, 28, 3403. https://doi.org/10.3390/molecules28083403
Ahmad, N., Ali, S., Abbas, M., Fazal, H., Saqib, S., Ali, A., Ullah, Z., Zaman, S., Sawati, L., Zada, A., & Sohail. (2023). Antimicrobial efficacy of Mentha piperata-derived biogenic zinc oxide nanoparticles against UTI-resistant pathogens. Scientific Reports, 13, 14972. https://doi.org/10.1038/s41598-023-41502-w
Ai, J., Biazar, E., Jafarpour, M., Montazeri, M., Majdi, A., Aminifard, S., Zafari, M., Akbari, H. R., & Rad, H. G. (2011). Nanotoxicology and nanoparticle safety in biomedical designs. International Journal of Nanomedicine, 6, 1117–1127. https://doi.org/10.2147/IJN.S16603
Gowramma, B., Keerthi, U., Rafi, M., & Rao, D. M. (2015). Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. Biotech, 5(2), 195–201. https://doi.org/10.1007/s13205-014-0210-4
Gour, A., & Jain, N. K. (2019). Advances in green synthesis of nanoparticles. Artificial Cells, Nanomedicine and Biotechnology, 9(47), 844–851. https://doi.org/10.1155/2023/9940845
Asghar, M., Habib, S., Zaman, W., Hussain, S., Ali, H., & Saqib, S. (2020). Synthesis and characterization of microbial mediated cadmium oxide nanoparticles. Microscopy Research and Technique, 83, 1574–1584. https://doi.org/10.1002/jemt.23553
Feroze, N., Arshad, B., Younas, M., Afridi, M. I., Saqib, S., & Ayaz, A. (2020). Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microscopy Research and Technique, 83, 72–80. https://doi.org/10.1002/jemt.23390
Saqib, S., Zaman, W., Ayaz, A., Habib, S., Bahadur, S., & S., Muhammad, S., & Ullah, F. (2020). Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatalysis and Agricultural Biotechnology, 28, 101729. https://doi.org/10.1016/j.bcab.2020.101729
Vijayan, S., Divya, K., Varghese, S., & Jisha, M. S. (2020). Antifungal efficacy of chitosan-stabilized biogenic silver nanoparticles against pathogenic Candida spp. Isolated from Human. BioNanoScience, 10, 974–982. https://doi.org/10.1007/s12668-020-00781-7
Zhang, X. F., Liu, Z. G., Shen, W., & Gurunathan, S. (2016). Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. International Journal of Molecular Sciences, 17(9), 1534. https://doi.org/10.3390/ijms17091534
Vijayaram, S., Razafindralambo, H., Sun, Y. Z., Vasantharaj, S., Ghafarifarsani, H., Hoseinifar, S. H., & Raeeszadeh, M. (2024). Applications of green synthesized metal nanoparticles — A review. Biological Trace Element Research, 202, 360–386. https://doi.org/10.1007/s12011-023-03645-9
Lima, S. L., Colombo, A. L., & de Almeida, J. N. (2019). Fungal cell wall: Emerging antifungals and drug resistance. Frontiers in Microbiology, 10, 2573. https://doi.org/10.3389/fmicb.2019.02573
Kowalczyk, P., Szymczak, M., Maciejewska, M., Laskowski, Ł., Laskowska, M., Ostaszewski, R., Skiba, G., & Franiak-Pietryga, I. (2021). All that glitters is not silver-A new look at microbiological and medical applications of silver nanoparticles. International Journal of Molecular Science, 22(2), 854. https://doi.org/10.3390/ijms22020854
Tosoni, N. F., Perini, H. F., Terra, M. R., Katsuda, M. S., Furlaneto, M. C., & Furlaneto-Maia, L. (2019). Antimicrobial activity of enterocin obtained from Enterococcus durans on Shiga-like toxin-producing Escherichia coli. Ciência Rural, 49(9), e20190297. https://doi.org/10.1590/0103-8478cr20190297
Rocha, K. R., Perini, H. F., de Souza, C. M., Schueler, J., Tosoni, N. F., Furlaneto, M. C., & Furlaneto-Maia, L. (2019). Inhibitory effect of bacteriocins from enterococci on developing and preformed biofilms of Listeria monocytogenes, Listeria ivanovii and Listeria innocua. World Journal of Microbiology and Biotechnology, 35, 96. https://doi.org/10.1007/s11274-019-2675-0
Furlaneto-Maia, L., Ramalho, R., Rocha, K. R., & Furlaneto, M. C. (2020). Antimicrobial activity of enterocins against Listeria sp. and other food spoilage bacteria. Biotechnology Letters, 42, 797–806. https://doi.org/10.1007/s10529-020-02810-7
Manikprabhu, D., Cheng, J., Chen, W., Sunkara, A. K., Mane, S. B., Kumar, R., Das, M., Hozzein, W. N., Duan, Y.-Q., & Li, W.-J. (2016). Sunlight mediated synthesis of silver nanoparticles by a novel actinobacterium (Sinomonas mesophila MPKL 26) and its antimicrobial activity against multi-drug resistant Staphylococcus aureus. Journal of Photochemistry & Photobiology, B: Biology, 158, 202–205. https://doi.org/10.1016/j.jphotobiol.2016.01.018
Phuc, L. D., Thien, V. H., & Dong, N. V. (2023). Analysis of silver nanoparticles by flame atomic absorption spectrometry. Vietnam Journal of Chemistry, 61, 109–117. https://doi.org/10.1002/vjch.202300086
Holder, I. A., & Neely, A. N. (2001). Does the addition of nystatin to 5% mafenide acetate and genitourinary irrigant solutions interfere with their antimicrobial activity? Assessment by two topical antimicrobial test assay systems. Journal of Burn Care & Rehabilitation, 22(4), 282–287. https://doi.org/10.1097/00004630-200107000-00007
EUCAST. (2020). EUCAST Antifungal MIC method for yeasts. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts; (E.DEF 7.3.2); EUCAST Development Laboratory for Fungi: Copenhagen, Denmark.
European Committee on Antimicrobial Susceptibility Testing. Fluconazole: Rationale for the clinical breakpoints, version 3.0, 2020. http://www.eucast.org. Accessed 01/03/2023
Guo, N., Ling, G., Liang, X., Jin, J., Fan, J., Qiu, J., Song, Y., Huang, N., Wu, X., Wang, X., Deng, X., Deng, X., & Yu, L. (2011). In vitro synergy of pseudolaric acid B and fluconazole against clinical isolates of Candida albicans. Mycoses, 54(5), 400–406. https://doi.org/10.1111/j.1439-0507.2010.01935.x
Odds, F. C. (2003). Synergy, antagonism, and what the chequerboard puts between them. Journal of Antimicrobial Chemotherapy, 52(1), 1. https://doi.org/10.1093/jac/dkg301
Abdel-Hadi, A., Iqbal, D., Alharbi, R., Jahan, S., Darwish, O., Alshehri, B., Banawas, S., Palanisamy, M., Ismail, A., Aldosari, S., Alsaweed, M., Madkhali, Y., Kamal, M., & Fatima, F. (2023). Myco-synthesis of silver nanoparticles and their bioactive role against pathogenic microbes. Biology, 12, 61. https://doi.org/10.3390/biology12050661
Abdallah, B. M., & Ali, E. M. (2022). Therapeutic effect of green synthesized silver nanoparticles using Erodium glaucophyllum extract against oral candidiasis: In vitro and in vivo study. Molecules, 27, 4221. https://doi.org/10.3390/molecules27134221
Johnson, M. B., & Criss, A. K. (2013). Fluorescence microscopy methods for determining the viability of bacteria in association with mammalian cells. Journal of visualized experiments, 79, 50729. https://doi.org/10.3791/50729
de Jesús Ruíz-Baltazar, Á., Reyes-López, S. Y., de Lourdes Mondragón-Sánchez, M., Estevez, M., Hernández-Martinez, A. R., & Pérez, R. (2018). Biosynthesis of Ag nanoparticles using Cynara cardunculus leaf extract: Evaluation of their antibacterial and electrochemical activity. Results in Physics, 11, 1142–1149. https://doi.org/10.1016/j.rinp.2018.11.032
Abdollahnia, M., Makhdoumi, A., Mashreghi, M., & Eshghi, H. (2020). Exploring the potentials of halophilic prokaryotes from a solar saltern for synthesizing nanoparticles: The case of silver and selenium. PLoS One, 15(3), e0229886. https://doi.org/10.1371/journal.pone.0229886
Safaei, M., Mozaffari, H. R., Moradpoor, H., Imani, M. M., Sharifi, R., & Golshah, A. (2022). Optimization of green synthesis of selenium nanoparticles and evaluation of their antifungal activity against oral Candida albicans infection. Advances in Materials Science and Engineering, 1376998. https://doi.org/10.1155/2022/1376998
Monowar, T., Rahman, M. S., Bhore, S. J., & Sathasivam, K. V. (2021). Endophytic bacteria Enterobacter hormaechei fabricated silver nanoparticles and their antimicrobial activity. Pharmaceutics, 13(4), 511. https://doi.org/10.3390/pharmaceutics13040511
Kumarasinghe, K. G. U. R., Silva, W. C. H., Fernando, M. D. A., Palliyaguru, L., Jayawardena, P. S., Shimomura, M., Fernando, S. S. N., Gunasekara, T. D. C. P., & Jayaweera, P. M. (2021). One-pot reducing agent-free synthesis of silver nanoparticles/nitrocellulose composite surface coating with antimicrobial and antibiofilm activities. Biomed Research International, 6666642. https://doi.org/10.1155/2021/6666642
Rai, M., Ingle, A. P., Trzcińska-Wencel, J., Wypij, M., Bonde, S., Yadav, A., Kratošová, G., & Golińska, P. (2021). Biogenic silver nanoparticles: What we know and what do we need to know? Nanomaterials, 11, 2901. https://doi.org/10.3390/nano11112901
Wu, Y., Pang, X., Wu, Y., Liu, X., & Zhang, X. (2022). Enterocins: Classification, synthesis, antibacterial mechanisms and food applications. Molecules, 27(7), 2258. https://doi.org/10.3390/molecules27072258
Sunkar, S., & Nachiyar, C. V. (2012). Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pacific Journal of Tropical Biomedicine, 2, 953–959. https://doi.org/10.1016/S2221-1691(13)60006-4
Saravanan, M., Barik, S. K., Ali, D. M., Prakash, P., & Pugazhendhi, A. (2018). Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microbial Pathogenesis, 116, 221–226. https://doi.org/10.1016/j.micpath.2018.01.038
Khan, T., Yasmin, A., & Townley, H. E. (2020). An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids and Surfaces B: Biointerfaces, 194, 111156. https://doi.org/10.1016/j.colsurfb.2020.111156
Jackson, T. C., Patani, B. O., & Israel, M. B. (2017). Nanomaterials and cell interactions: A review. Journal of Biomaterials and Nanobiotechnology, 8, 220–228. https://doi.org/10.4236/jbnb.2017.84015
De Souza, A. O. (2022). Overview of nanomaterials and cellular interactions. Biointerface Research in Applied Chemistry, 13, 367. https://doi.org/10.33263/BRIAC134.367
Pereira, G. H., Müller, P. R., Szeszs, M. W., Levin, A. S., & Melhem, M. S. C. (2010). Five-year evaluation of bloodstream yeast infections in a tertiary hospital: The predominance of non-C. albicans Candida species. Medical Mycology, 48(6), 839–842. https://doi.org/10.3109/13693780903580121
Arshad, F., Naikoo, G. A., Hassan, I. U., Chava, S. R., Tanani, M. E., Aljabali, A. A., & Tambuwala, M. M. (2023). Bioinspired and green synthesis of silver nanoparticles for medical applications: A green perspective. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-023-04719-z
Ahmad, A., Wei, Y., Syed, F., Tahir, K., Khan, A. U., Hameed, M. U., & Yuan, Q. (2016). Amphotericin B-conjugated biogenic silver nanoparticles as an innovative strategy for fungal infections. Microbial Pathogenesis, 99, 271–281. https://doi.org/10.1016/j.micpath.2016.08.031
Soliman, A. M., Abdel-Latif, W., Shehata, I. H., Fouda, A., Abdo, A. M., & Ahmed, Y. M. (2021). Green approach to overcome the resistance pattern of Candida spp. using biosynthesized silver nanoparticles fabricated by Penicillium chrysogenum F9. Biological Trace Element Research, 199, 800–811. https://doi.org/10.1007/s12011-020-02188-7
Guerra, J. D., Sandoval, G., Avalos-Borja, M., Pestryakov, A., Garibo, D., Susarrey-Arce, A., & Bogdanchikova, N. (2020). Selective antifungal activity of silver nanoparticles: A comparative study between Candida tropicalis and Saccharomyces boulardii. Colloid and Interface Science Communications, 37, 100280. https://doi.org/10.1016/j.colcom.2020.100280
Acknowledgements
EAP and NAAS were fellowship holders of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES). MCF is grateful to CNPq for the PQ fellowship.
Funding
This work was supported by Fundação Araucária/SETI/Governo do Paraná–Brazil and PROPPG/UEL-Brazil (Project 13349). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Author information
Authors and Affiliations
Contributions
EAP and MCF conceived and designed the experiments. EAP, CMS, and NAAS performed the experiments. JNQ formal analysis. EAP and MCF analyzed the data and drafted the manuscript. EAP, LF-M, and MCF reviewed and edited the manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for Publication
Not applicable.
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paulo, E.A., de Souza, C.M., de Souza, N.A.A. et al. One-Pot and Environmentally Friendly Biosynthesis of Silver Nanoparticles from Enterococcus durans: Activity Against Fluconazole-Resistant Pathogenic Candida tropicalis. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01336-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01336-w