Abstract
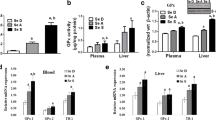
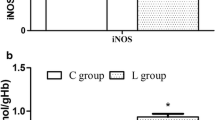
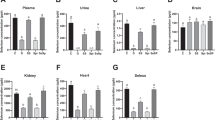
Selenium (Se) is an essential trace element that maintains normal physiological functions in organisms. Since the discovery of glutathione peroxidase (GSH-PX), public interest in selenoproteins has gradually increased. Based on previous studies, dietary Se maintains erythrocyte homeostasis through selenoprotein-induced mediation of redox reactions. Furthermore, both the surface phosphatidylserine (PS) and intramembrane stomatin contents can be used as indicators of erythrocyte osmotic fragility. This study focused on the mechanism by which dietary Se deficiency increases erythrocyte osmotic fragility. We fed Se-deficient grain to mice for 8 weeks to establish a Se deficiency model in mice. We measured Se levels in the blood as well as the activities of antioxidant enzymes associated with selenoproteins in a Se-deficient environment. We used Western blotting, routine blood analysis, and other methods to detect red blood cell oxidative stress levels, membrane stomatin levels, and PS externalization. Fresh blood was collected to test erythrocyte osmotic fragility. The results showed that antioxidant enzyme activity was affected by dietary Se deficiency. Oxidative stress increased lipid peroxidation and the ROS content in the blood of the mice. Under such conditions, decreased PS exposure and stomatin content in the erythrocyte membrane eventually affected the structure of the erythrocyte membrane and increased erythrocyte osmotic fragility.




Similar content being viewed by others
References
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241
Zhang Z, Liu Q, Yang J, Yao H, Fan R, Cao C, Liu C, Zhang S, Xin GL, Xu S (2020) Proteomic profiling of multiple tissue damage in chickens for selenium deficiency biomarker discovery. Food Funct 11:1312–1321. https://doi.org/10.1039/C9FO02861G
Wang N, Tan HY, Li S, Xu Y, Guo W, Feng Y (2017) Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxidative Med Cell Longev 2017:7478523
Wang FH, Peng X, Chen Y, Wang Y, Yang M, Guo MY (2019) Se regulates the contractile ability of uterine smooth Musclevia Selenoprotein N, Selenoprotein T, and Selenoprotein Win mice. Biol Trace Elem Res 192(2):196–205
Nishi K, Ferguson LR, John T, Jonathan M (2006) Hemolysate thioredoxin reductase and glutathione peroxidase activities correlate with serum selenium in a group of New Zealand men at high prostate cancer risk. J Nutr 136(8):2232–2235
Dalto DB, Matte JJ (2017) Pyridoxine (vitamin B(6)) and the glutathione peroxidase system; a link between one-carbon metabolism and antioxidation. Nutrients 9(3):e189
Yang J, Gong Y, Cai J, Liu Q, Zhang Z (2019) Lnc-3215 suppression leads to calcium overload in selenium deficiency-induced chicken heart lesion via the lnc-3215-miR-1594-TNN2 pathway. Mol Ther Nucleic Acids 18:1–15
Alehagen U, Aaseth J (2015) Selenium and coenzyme Q10 interrelationship in cardiovascular diseases--a clinician's point of view. J Trace Elem Med Biol 31:157–162
Karimi F, Omrani GR (2019) Effects of selenium and vitamin C on the serum level of antithyroid peroxidase antibody in patients with autoimmune thyroiditis. J Endocrinol Investig 42(4):481–487
Filippini T, Michalke B, Wise LA (2018) Diet composition and serum levels of selenium species: a cross-sectional study. Food Chem Toxicol 115:482–490
Liu H, Yu F, Shao W (2018) Associations between selenium content in hair and Kashin-Beck disease/Keshan disease in children in northwestern China: a prospective cohort study. Biol Trace Elem Res 184(1):16–23
Maiyo F, Singh M (2017) Selenium nanoparticles: potential in cancer gene and drug delivery. Nanomedicine 12(9):1075–1089
Nordestgaard BG, Langsted A (2016) Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res 57(11):1953–1975
Liu J, Wang S, Zhang Q, Li X, Xu S (2020) Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3pp38 MAPK axis and oxidative stress. Metallomics 12(1):54–64
Hu X, Tan S, Yin H, Khoso PA, Xu Z, Li S (2020) Selenium-mediated gga-miR-29a-3p regulates LMH cell proliferation, invasion, and migration by targeting COL4A2. Metallomics 12:449–459. https://doi.org/10.1039/c9mt00266a
Kaur R, Ghanghas P, Rastogi P, Kaushal N (2019) Protective role of selenium against hemolytic anemia is mediated through redox modulation. Biol Trace Elem Res 189(2):490–500
Dhaliwal G, Cornett PA, Tierney L Jr (2004) Hemolytic anemia. Am Fam Physician 69(11):2599–2606
Guillaud C, Loustau V, Michel M (2012) Hemolytic anemia in adults: main causes and diagnostic procedures. Expert Rev Hematol 5(2):229–241
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524:13–30
Slatter DA, Bolton CH, Bailey AJ (2000) The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia 43(5):550–557
Semba RD, Ricks MO, Ferrucci L, Xue QL, Guralnik JM, Fried LP (2009) Low serum selenium is associated with anemia among older adults in the United States. Eur J Clin Nutr 63(1):93–99
Roland K, Msamanga GI, Donna S (2004) Selenium status is associated with accelerated HIV disease progression among HIV-1-infected pregnant women in Tanzania. J Nutr 134(10):2556–2560
Salzer U, Zhu R, Luten M (2008) Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion 48(3):451–462
Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS (2007) Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion 47(7):1212–1220
Casadei BR, Domingues CC, de Paula E, Riske KA (2014) Direct visualization of the action of Triton X-100 on giant vesicles of erythrocyte membrane lipids. Biophys J 106(11):2417–2425
Hancock JF (2006) Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7(6):456–462
Sabina RL, Wandersee NJ, Hillery CA (2009) Ca2+-CaM activation of AMP deaminase contributes to adenine nucleotide dysregulation and phosphatidylserine externalization in human sickle erythrocytes. Br J Haematol 144(3):434–445
Orbach A, Zelig O, Yedgar S, Barshtein G (2017) Biophysical and biochemical markers of red blood cell fragility. Transfus Med Hemother 44(3):183–187
Liao C, Carlson BA, Paulson RF, Prabhu KS (2018) The intricate role of selenium and selenoproteins in erythropoiesis. Free Radic Biol Med 127:165–171
Soares JC, Folmer V, Da Rocha JB, Nogueira CW (2014) Ebselen exhibits glycation-inhibiting properties and protects against osmotic fragility of human erythrocytes in vitro. Cell Biol Int 38(5):625–630
Salem NY, Yehia SG, Farag HS, Elkhiat MA (2016) Clinical, hemato-biochemical alterations and oxidant-antioxidant biomarkers in Babesia-infected calves. Int J Vet Sci Med 4(1):17–22
Lykkesfeldt J (2007) Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta 380(1–2):50–58
Bassé F, Stout JG, Sims PJ, Wiedmer T (1996) Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem 271(29):17205–17210
Chen Y, Ji LL, Liu TY, Wang ZT (2011) Evaluation of gender-related differences in various oxidative stress enzymes in mice. Chin J Phys 54(6):385–390
Liang Q, Sheng Y, Jiang P, Ji L, Xia Y, Min Y, Wang Z (2011) The gender-dependent difference of liver GSH antioxidant system in mice and its influence on isoline-induced liver injury. Toxicology 280(1–2):61–69
Biernatowska A, Augoff K, Podkalicka J (2017) MPP1 directly interacts with flotillins in erythrocyte membrane - possible mechanism of raft domain formation. Biochim Biophys Acta Biomembr 1859(11):2203–2212
Card RT, Mohandas N, Mollison PL (2010) Relationship of post-transfusion viability to deformability of stored red cells. Br J Haematol 53(2):237–240
Willekens FL, Werre JM, Groenen-Dopp YA, Roerdinkholder-Stoelwinder B, de Pauw B, Bosman GJ (2008) Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol 141(4):549–556
Bosman GJCGM, Lasonder E, Groenen-Döpp YAM, Willekens FLA, Werre JM, Novotný VMJ (2010) Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteome 73(3):396–402
Ansari SA, Pendurthi UR, Sen P, Rao LV (2016) The role of putative phosphatidylserine-interactive residues of tissue factor on its coagulant activity at the cell surface. PLoS One 11(6):e0158377
Barshtein G, Gural A, Manny N, Zelig O, Yedgar S, Arbell D (2014) Storage-induced damage to red blood cell mechanical properties can be only partially reversed by rejuvenation. Transfus Med Hemother 41(3):197–204
Malka R, Delgado FF, Manalis SR, Higgins JM (2014) In vivo volume and hemoglobin dynamics of human red blood cells. PLoS Comput Biol 10(10):e1003839
Doyle RL, Da Silva AS, Oliveira CB (2016) Cholinesterases as markers of the inflammatory process associated oxidative stress in cattle infected by Babesia bigemina. Comp Immunol Microbiol Infect Dis 46:1–6
Li Y, Yu S, Gan X (2017) MRP-1 and BCRP promote the externalization of phosphatidylserine in oxalate-treated renal epithelial cells: implications for calcium oxalate urolithiasis. Urology 107:271 e9–271.e17
Langhorst MF, Solis GP, Hannbeck S, Plattner H, Stuermer CA (2007) Linking membrane microdomains to the cytoskeleton: regulation of the lateral mobility of reggie-1/flotillin-2 by interaction with actin. FEBS Lett 581(24):4697–4703
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, Sy., Chen, Sj., Liang, W. et al. Dietary Selenium Deficiency Facilitated Reduced Stomatin and Phosphatidylserine Externalization, Increasing Erythrocyte Osmotic Fragility in Mice. Biol Trace Elem Res 199, 594–603 (2021). https://doi.org/10.1007/s12011-020-02162-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02162-3