Abstract
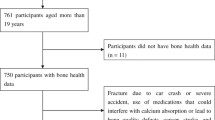
Osteoporosis and its consequence of fragility fracture represent a major public health problem. Human exposure to heavy metals has received considerable attention over the last decades. However, little is known about the influence of co-exposure to multiple heavy metals on bone density. The present study aimed to examine the association between exposure to metals and bone mineral density (BMD) loss. Blood and urine concentrations of 20 chemical elements were selected from 3 cycles (2005–2010) NHANES (National Health and Nutrition Examination Survey), in which we included white women over 50 years of age and previously selected for BMD testing (N = 1892). The bone loss group was defined as participants having T-score < − 1.0, and the normal group was defined as participants having T-score ≥ − 1.0. We developed classification models based on support vector machines capable of determining which factors could best predict BMD loss. The model which included the five-best features-selected from the random forest were age, body mass index, urinary concentration of arsenic (As), cadmium (Cd), and tungsten (W), which have achieved high scores for accuracy (92.18%), sensitivity (90.50%), and specificity (93.35%). These data demonstrate the importance of these factors and metals to the classification since they alone were capable of generating a classification model with a high prediction of accuracy without requiring the other variables. In summary, our findings provide insight into the important, yet overlooked impact that arsenic, cadmium, and tungsten have on overall bone health.



Similar content being viewed by others
References
Cooper C, Campion G, Melton LJ (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2:285–289
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287. https://doi.org/10.1016/S0140-6736(10)62349-5
Compston JE, Mcclung MR, Leslie WD (2019) Osteoporosis. Lancet 393:364–376. https://doi.org/10.1016/S0140-6736(18)32112-3
Boyanov M (2009) The diagnosis of osteoporosis and the fracture risk assessment. Endokrinologya 14:30–36
ClinRisk. Welcome to the QFracture-2016 risk calculator. https://qfracture.org/. Accessed 15 Oct 2019
Garvan Institute. Bone fracture risk calculator. https://www.garvan.org.au/bone-fracture-risk. Accessed 15 Oct 2019
University of Sheffield. Fracture risk assessment tool. https://www.sheffield.ac.uk/FRAX/. Accessed 15 Oct 2019
Agency for Toxic Substances and Disease Registry (ATSDR) (2017) Substance priority list. https://www.atsdr.cdc.gov/SPL/. Accessed 3 Jan 2020
Schümann K, Elsenhans B (2002) The impact of food contaminants on the bioavailability of trace metals. J Trace Elem Med Biol 16:139–144
Milnerowicz H, Ściskalska M, Dul M (2015) Pro-inflammatory effects of metals in persons and animals exposed to tobacco smoke. J Trace Elem Med Biol 29:1–10
Kim JJ, Kim YS, Kumar V (2019) Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elem Med Biol 54:226–231
Rodríguez J, Mandalunis PM (2018) A review of metal exposure and its effects on bone health. J Toxicol 2018:. https://doi.org/10.1155/2018/4854152
Brzóska MM, Moniuszko-Jakoniuk J (2005) Disorders in bone metabolism of female rats chronically exposed to cadmium. Toxicol Appl Pharmacol 202:68–83. https://doi.org/10.1016/j.taap.2004.06.007
Brzóska MM, Moniuszko-Jakoniuk J (2004) Low-level exposure to cadmium during the lifetime increases the risk of osteoporosis and fractures of the lumbar spine in the elderly: studies on a rat model of human environmental exposure. Toxicol Sci 82:468–477. https://doi.org/10.1093/toxsci/kfh275
Wallin M, Barregard L, Sallsten G, Lundh T, Karlsson MK, Lorentzon M, Ohlsson C, Mellström D (2016) Low-level cadmium exposure is associated with decreased bone mineral density and increased risk of incident fractures in elderly men: the MrOS Sweden Study. J Bone Miner Res 31:732–741. https://doi.org/10.1002/jbmr.2743
Engström A, Michaëlsson K, Vahter M, Julin B, Wolk A, Åkesson A (2012) Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 50:1372–1378. https://doi.org/10.1016/j.bone.2012.03.018
Wani AL, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8:55–64
Wong AKO, Beattie KA, Bhargava A, Cheung M, Webber CE, Chettle DR, Papaioannou A, Adachi JD, Canadian Multicentre Osteoporosis Study (CaMos) Research Group (2015) Bone lead (Pb) content at the tibia is associated with thinner distal tibia cortices and lower volumetric bone density in postmenopausal women. Bone 79:58–64. https://doi.org/10.1016/j.bone.2015.05.010
Levin R, Brown MJ, Kashtock ME, Jacobs DE, Whelan EA, Rodman J, Schock MR, Padilla A, Sinks T (2008) Lead exposures in U.S. children, 2008: implications for prevention. Environ. Health Perspect 116:1285–1293
Bruno A. Rocha, Alexandros G. Asimakopoulos, Masato Honda, Nattane L. da Costa, Rommel M. Barbosa, Fernando Barbosa Jr KK (2017) Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage. Environ Sci Technol
Park SK, Zhao Z, Mukherjee B (2017) Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ Health 16:1–17. https://doi.org/10.1186/s12940-017-0310-9
Kupsco A, Kioumourtzoglou MA, Just AC, Amarasiriwardena C, Estrada-Gutierrez G, Cantoral A, Sanders AP, Braun JM, Svensson K, Brennan KJM, Oken E, Wright RO, Baccarelli AA, Téllez-Rojo MM (2019) Prenatal metal concentrations and childhood cardiometabolic risk using Bayesian kernel machine regression to assess mixture and interaction effects. Epidemiology 30:263–273. https://doi.org/10.1097/EDE.0000000000000962
Centers for Disease Control and Prevention (CDC) NC for HS (NCHS). National Health and Nutrition Examination Survey Data, 2009–2010. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2009. Accessed 3 Jan 2020
Centers for Disease Control and Prevention (CDC) NC for HS (NCHS). National Health and Nutrition Examination Survey Data, 2007–2008. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2007. Accessed 3 Jan 2020
Centers for Disease Control and Prevention (CDC) NC for HS (NCHS). National Health and Nutrition Examination Survey Data, 2005–2006. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2005. Accessed 3 Jan 2020
Date AR, Gray AL (1989) Applications of inductively coupled plasma mass spectrometry. Blackie
Kursa MB, Rudnicki WR (2010) Feature selection with the boruta package. J Stat Softw 36:1–13. https://doi.org/10.18637/jss.v036.i11
R Core Team (2013) R: a language and environment for statistical computing. R Found. Stat. Comput
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20:273–297. https://doi.org/10.1007/bf00994018
Noble WS (2006) What is a support vector machine? Nat Biotechnol 24:1565–1567. https://doi.org/10.1038/nbt1206-1565
Lo YC, Rensi SE, Torng W, Altman RB (2018) Machine learning in chemoinformatics and drug discovery. Drug Discov Today 23:1538–1546. https://doi.org/10.1016/j.drudis.2018.05.010
Porter SE, Hanley EN (2001) The musculoskeletal effects of smoking. J Am Acad Orthop Surg 9:9–17
Ferrari SL, Abrahamsen B, Napoli N et al (2018) Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 29:2585–2596
Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM, LEAP Study Group (2005) Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma 19:151–157. https://doi.org/10.1097/00005131-200503000-00001
Santiago HAR, Zamarioli A, Sousa Neto MD, Volpon JB (2017) Exposure to secondhand smoke impairs fracture healing in rats. Clin Orthop Relat Res 475:894–902. https://doi.org/10.1007/s11999-016-5184-6
Ren X, Mchale CM, Skibola CF et al (2011) An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect 119:11–19
Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121:295–302
World Health Organization (WHO) (2018) Guidelines for drinking-water quality, 3rd edition: volume 1 - recommendations. World Health Organization
Dani SU (2013) Osteoresorptive arsenic intoxication. Bone 53:541–545. https://doi.org/10.1016/j.bone.2013.01.017
Hu YC, Cheng HL, Hsieh BS, Huang LW, Huang TC, Chang KL (2012) Arsenic trioxide affects bone remodeling by effects on osteoblast differentiation and function. Bone 50:1406–1415. https://doi.org/10.1016/j.bone.2012.03.012
Marty M, Noirrit-Esclassan E, Diemer F (2016) Arsenic trioxide-induced osteo-necrosis treatment in a child: mini-review and case report. Eur Arch Paediatr Dent 17:419–422. https://doi.org/10.1007/s40368-016-0250-z
Dumlu A, Yalcinkaya S, Olgac V, Güvercin M (2007) Osteomyelitis due to arsenic trioxide use for tooth deviatlizaion. Int Endod J 40:317–322. https://doi.org/10.1111/j.0143-2885.2007.01230.x
Lever JH (2002) Paget’s disease of bone in Lancashire and arsenic pesticide in cotton mill wastewater: a speculative hypothesis. Bone 31:434–436. https://doi.org/10.1016/S8756-3282(02)00833-5
Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A (2017) The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect 125:087001
Kuo CC, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA, Goessler W, Lee E, Guallar E, Navas-Acien A (2015) Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care 38:620–627. https://doi.org/10.2337/dc14-1641
Wang SL, Chiou JM, Chen CJ, Tseng CH, Chou WL, Wang CC, Wu TN, Chang LW (2003) Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environ Health Perspect 111:155–159. https://doi.org/10.1289/ehp.5457
Lai MS, Hsueh YM, Chen CJ, Shyu MP, Chen SY, Kuo TL, Wu MM, Tai TY (1994) Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol 139:484–492. https://doi.org/10.1093/oxfordjournals.aje.a117031
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195–214
Heilmeier U, Cheng K, Pasco C, Parrish R, Nirody J, Patsch JM, Zhang CA, Joseph GB, Burghardt AJ, Schwartz AV, Link TM, Kazakia G (2016) Cortical bone laminar analysis reveals increased midcortical and periosteal porosity in type 2 diabetic postmenopausal women with history of fragility fractures compared to fracture-free diabetics. Osteoporos Int 27:2791–2802. https://doi.org/10.1007/s00198-016-3614-7
Dong XN, Qin A, Xu J, Wang X (2011) In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone 49:174–183. https://doi.org/10.1016/j.bone.2011.04.009
Rhee EJ, Kim YC, Lee WY, Jung CH, Sung KC, Ryu SH, Oh KW, Kim SW (2006) Comparison of insulin resistance and serum high-sensitivity C-reactive protein levels according to the fasting blood glucose subgroups divided by the newly recommended criteria for fasting hyperglycemia in 10 059 healthy Koreans. Metabolism 55:183–187. https://doi.org/10.1016/j.metabol.2005.08.010
Qu Y, Kang MY, Dong RP, Zhao JW (2016) Correlations between abnormal glucose metabolism and bone mineral density or bone metabolism. Med Sci Monit 22:824–832. https://doi.org/10.12659/MSM.895387
Douillet C, Currier J, Saunders J, Bodnar WM, Matoušek T, Stýblo M (2013) Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol 267:11–15. https://doi.org/10.1016/j.taap.2012.12.007
Cui Q, Fu J, Hu Y, Li Y, Yang B, Li L, Sun J, Chen C, Sun G, Xu Y, Zhang Q, Pi J (2017) Deficiency of long isoforms of Nfe2l1 sensitizes MIN6 pancreatic β cells to arsenite-induced cytotoxicity. Toxicol Appl Pharmacol 329:67–74. https://doi.org/10.1016/j.taap.2017.05.013
Dover EN, Beck R, Huang MC, Douillet C, Wang Z, Klett EL, Stýblo M (2018) Arsenite and methylarsonite inhibit mitochondrial metabolism and glucose-stimulated insulin secretion in INS-1 832/13 β cells. Arch Toxicol 92:693–704. https://doi.org/10.1007/s00204-017-2074-y
Shirakawa J, Terauchi Y (2014) Selective and sequential loss of transcriptional factors: a hallmark of β-cell failure in type 2 diabetes? J Diabetes Investig 5:359–361
Gibert Y, Cavarape A, Hesselson D, et al (2019) Braving the element: pancreatic β-cell dysfunction and adaptation in response to arsenic exposure. Front Endocrinol | www.frontiersin.org 1:344. https://doi.org/10.3389/fendo.2019.00344
Sun Q, Yang Q, Xu H, Xue J, Chen C, Yang X, Gao X, Liu Q (2019) MiR-149 negative regulation of mafA is involved in the arsenite-induced dysfunction of insulin synthesis and secretion in pancreatic beta cells. Toxicol Sci 167:4–125. https://doi.org/10.1093/toxsci/kfy150
Lee WC, Guntur AR, Long F, Rosen CJ (2017) Energy metabolism of the osteoblast: implications for osteoporosis. Endocr Rev 38:255–266
Tawfik DS, Viola RE (2011) Arsenate replacing phosphate: alternative life chemistries and ion promiscuity. Biochemistry 50:1128–1134. https://doi.org/10.1021/bi200002a
Dixon HBF (1996) The biochemical action of arsonic acids especially as phosphate analogues. Adv Inorg Chem 44:191–227. https://doi.org/10.1016/S0898-8838(08)60131-2
Sabir S, Akash MSH, Fiayyaz F, Saleem U, Mehmood MH, Rehman K (2019) Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: inserting the association into perspectives. Biomed Pharmacother 114:108802
Santra A, Maiti A, Chowdhury A, Mazumder DN (2000) Oxidative stress in liver of mice exposed to arsenic-contaminated water. Indian J Gastroenterol 19
Reyes-Hinojosa D, Lozada-Pérez CA, Zamudio Cuevas Y, López-Reyes A, Martínez-Nava G, Fernández-Torres J, Olivos-Meza A, Landa-Solis C, Gutiérrez-Ruiz MC, Rojas del Castillo E, Martínez-Flores K (2019) Toxicity of cadmium in musculoskeletal diseases. Environ Toxicol Pharmacol 72:103219. https://doi.org/10.1016/j.etap.2019.103219
Rodríguez J, Mandalunis PM (2016) Effect of cadmium on bone tissue in growing animals. Exp Toxicol Pathol 68:391–397. https://doi.org/10.1016/j.etp.2016.06.001
Almeida JA, Novelli ELB, Dal Pai Silva M, Alves Júnior R (2001) Environmental cadmium exposure and metabolic responses of the Nile tilapia, Oreochromis niloticus. Environ Pollut 114:169–175. https://doi.org/10.1016/S0269-7491(00)00221-9
Kraus T, Schramel P, Schaller KH et al (2001) Exposure assessment in the hard metal manufacturing industry with special regard to tungsten and its compounds. Occup Environ Med 58:631–634. https://doi.org/10.1136/oem.58.10.631
Bolt AM, Mann KK (2016) Tungsten: an emerging toxicant, alone or in combination. Curr Environ Health Rep 3:405–415. https://doi.org/10.1007/s40572-016-0106-z
EPA (2017) Technical fact sheet – tungsten. In: EPA 505-F-070- 005
Bolt AM, Grant MP, Wu TH, Flores Molina M, Plourde D, Kelly ADR, Negro Silva LF, Lemaire M, Schlezinger JJ, Mwale F, Mann KK (2016) Tungsten promotes sex-specific adipogenesis in the bone by altering differentiation of bone marrow-resident mesenchymal stromal cells. Toxicol Sci 150:333–346. https://doi.org/10.1093/toxsci/kfw008
Funding
This study was funded by the São Paulo Research Foundation (FAPESP, no 2018/24069-3).
Author information
Authors and Affiliations
Contributions
JPBX, AZ, and FBJ designed the study. JPBX performed the analyses. JPBX, AZ, and FBJ analyzed data and wrote the manuscript. JPBX, AZ, MK, RMB, and FBJ interpreted the data and revised the manuscript. All authors approve of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ximenez, J.P.B., Zamarioli, A., Kacena, M.A. et al. Association of Urinary and Blood Concentrations of Heavy Metals with Measures of Bone Mineral Density Loss: a Data Mining Approach with the Results from the National Health and Nutrition Examination Survey. Biol Trace Elem Res 199, 92–101 (2021). https://doi.org/10.1007/s12011-020-02150-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02150-7